Preparation method of inactivated porcine parvovirus vaccine and product thereof
A technology for parvovirus and inactivated vaccine, which is applied in the directions of medical preparations, antiviral agents, and pharmaceutical formulations containing active ingredients, which can solve the problems of low porcine parvovirus liquid culture yield, unstable vaccine quality, and difficulty in scaling up. , to achieve the effect of improving the yield and quality of vaccines, maintaining a long time for cells, and achieving a balanced and stable quality
Active Publication Date: 2011-06-15
哈药集团生物疫苗有限公司
View PDF3 Cites 12 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
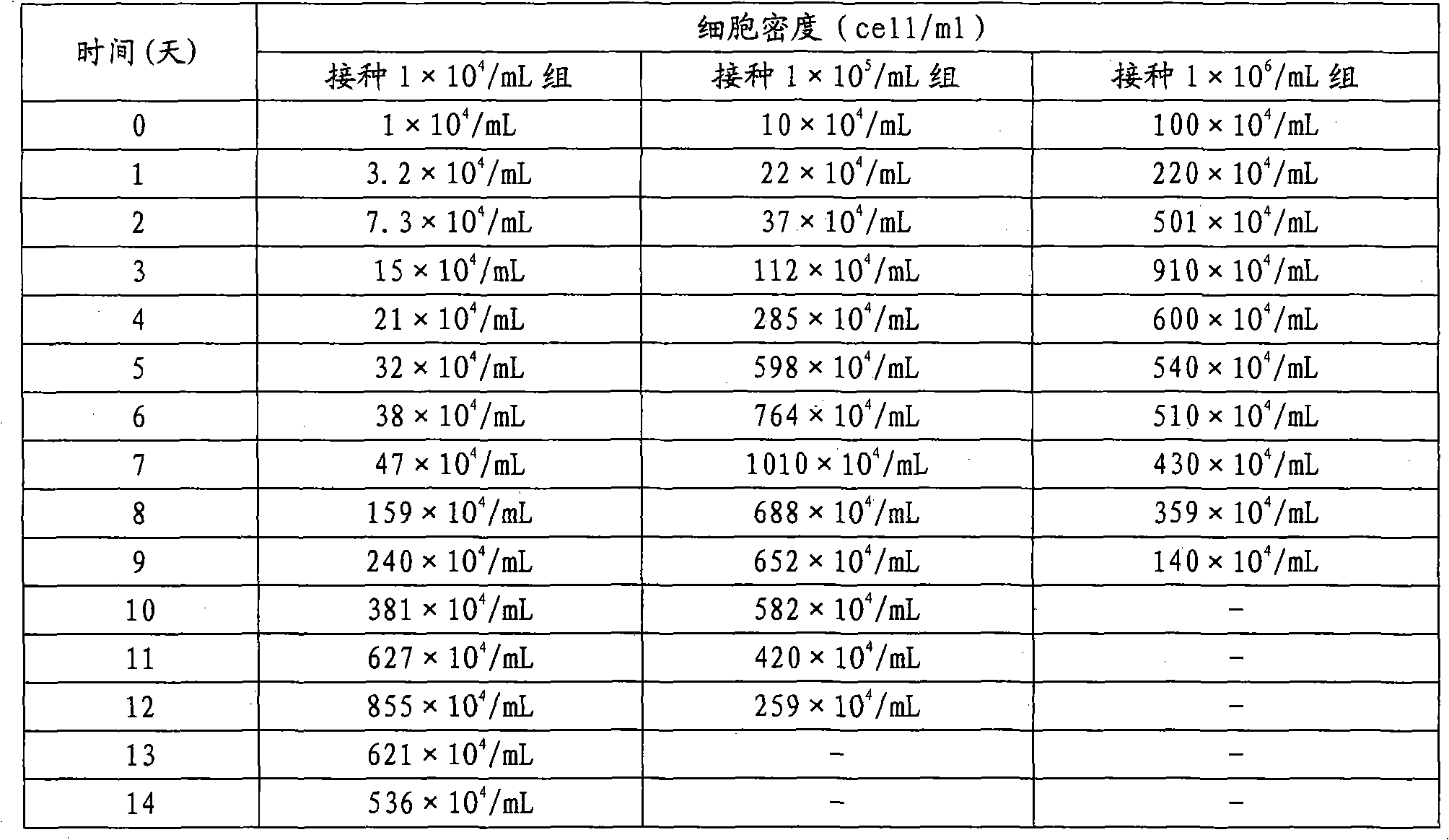
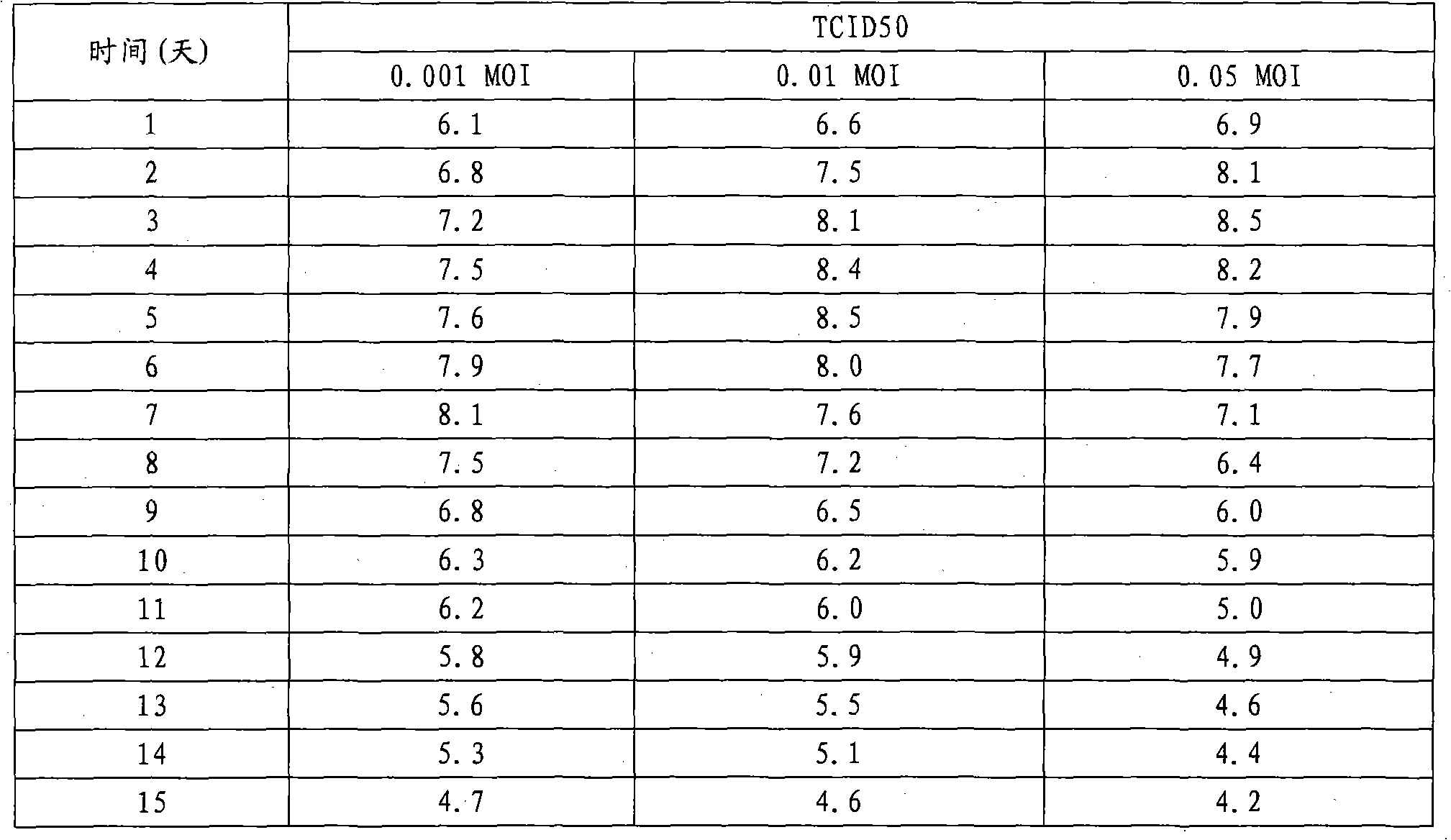
The technical problem to be solved by the present invention is to overcome the defects such as low porcine parvovirus liquid culture yield, difficult scale expansion, high cost and unstable vaccine quality in the existing preparation method of porcine parvovirus inactivated vaccine, and provide A new preparation method of porcine parvovirus inactivated vaccine. The preparation method utilizes microcarrier suspension culture ST cells in bioreactor to efficiently produce porcine parvovirus liquid. The production process parameters are optimized. The method has the advantages of porcine parvovirus produced The virus liquid has the advantages of high virus titer, large-scale industrial production, low cost, and stable vaccine quality.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
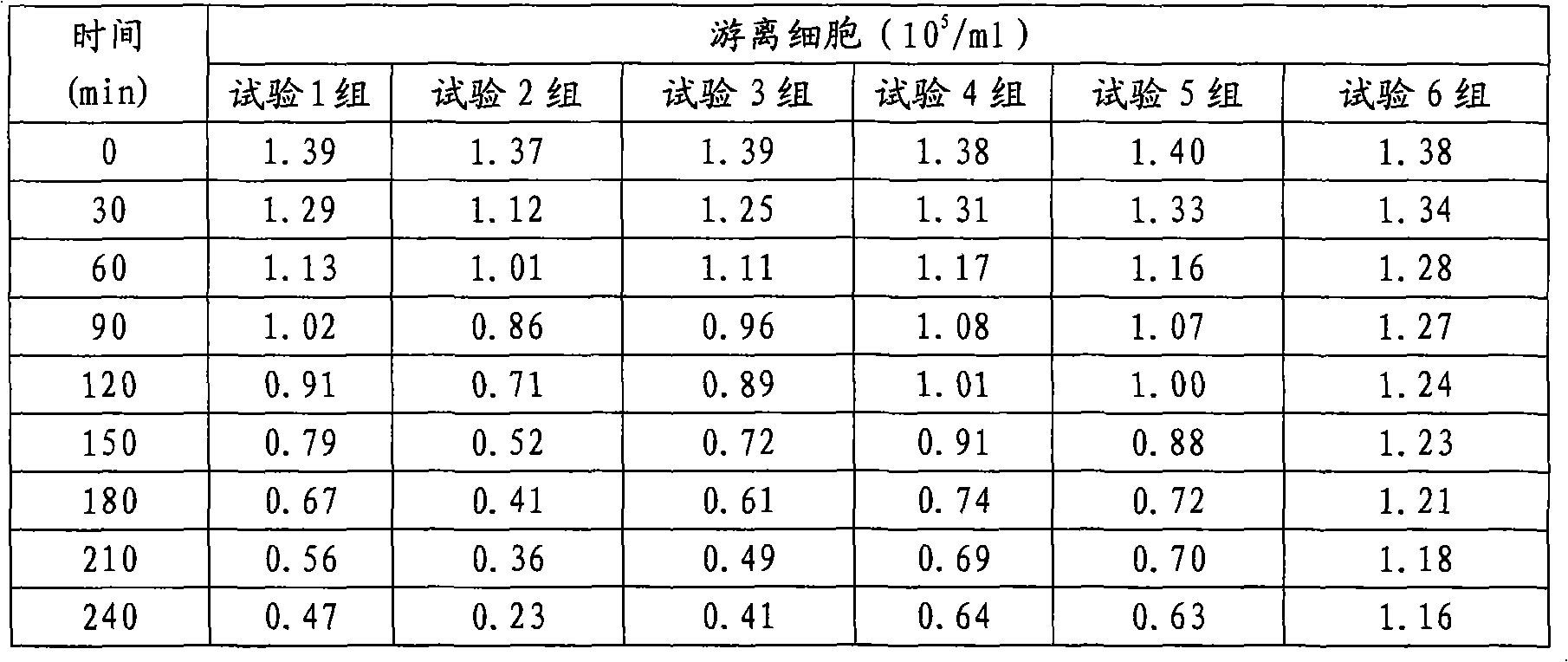
The invention discloses a preparation method of inactivated porcine parvovirus vaccine, comprising the following steps: (1) inoculating ST (swine testicle) cells with lentogen strains of porcine parvovirus, culturing to obtain production virus seeds; (2) adding treated microcarriers to a cell growth medium in a bioreactor, stirring; (3) inoculating the ST cells into the microcarriers in the bioreactor for cell reproduction and culturing; (4) infecting the suspension cultured ST cells in the bioreactor with the production virus seeds of the porcine parvovirus for virus reproduction and culturing; and (5) gathering the reproduced virus liquid, inactivating and preparing the vaccine. The method provided by the invention has long cell hold time, high cell viability and high density of living cells, and is favorable for continuous reproduction of virus, so that the prepared virus liquid has high virus titer. The production processes of the method disclosed by the invention are tightly and smoothly linked, the production scale is easy to enlarge, the porcine parvovirus is convenient to gather and stable in quality, and the method can obviously lower the production cost and effectively improve the yield and quality of the vaccine.
Description
Preparation method and product of porcine parvovirus inactivated vaccine technical field The invention relates to a preparation method of a veterinary live vaccine, in particular to a preparation method of an inactivated porcine parvovirus vaccine and an inactivated porcine parvovirus vaccine prepared by the method, belonging to the field of preparation of an inactivated porcine parvovirus vaccine. Background technique Porcine parvoviurs (PPV) can cause reproductive disorders in pigs, mainly manifested as infection and death of fetuses and embryos, while the mother usually does not show clinical symptoms. Serologically negative sows are primarily infected via the oronasal route during the first half of gestation, with consequent transplacental infection of immunocompromised fetuses leading to disease. The virus is ubiquitous in pig herds around the world and endemic in most farms. The disease is very popular and has been reported in many countries in Europe, America, Asia...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61P31/20A61K39/23
Inventor 姜力藏玉婷柴华王彬王琪任德强戴秀莉张智明武佳斌张明艳张婷婷
Owner 哈药集团生物疫苗有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com