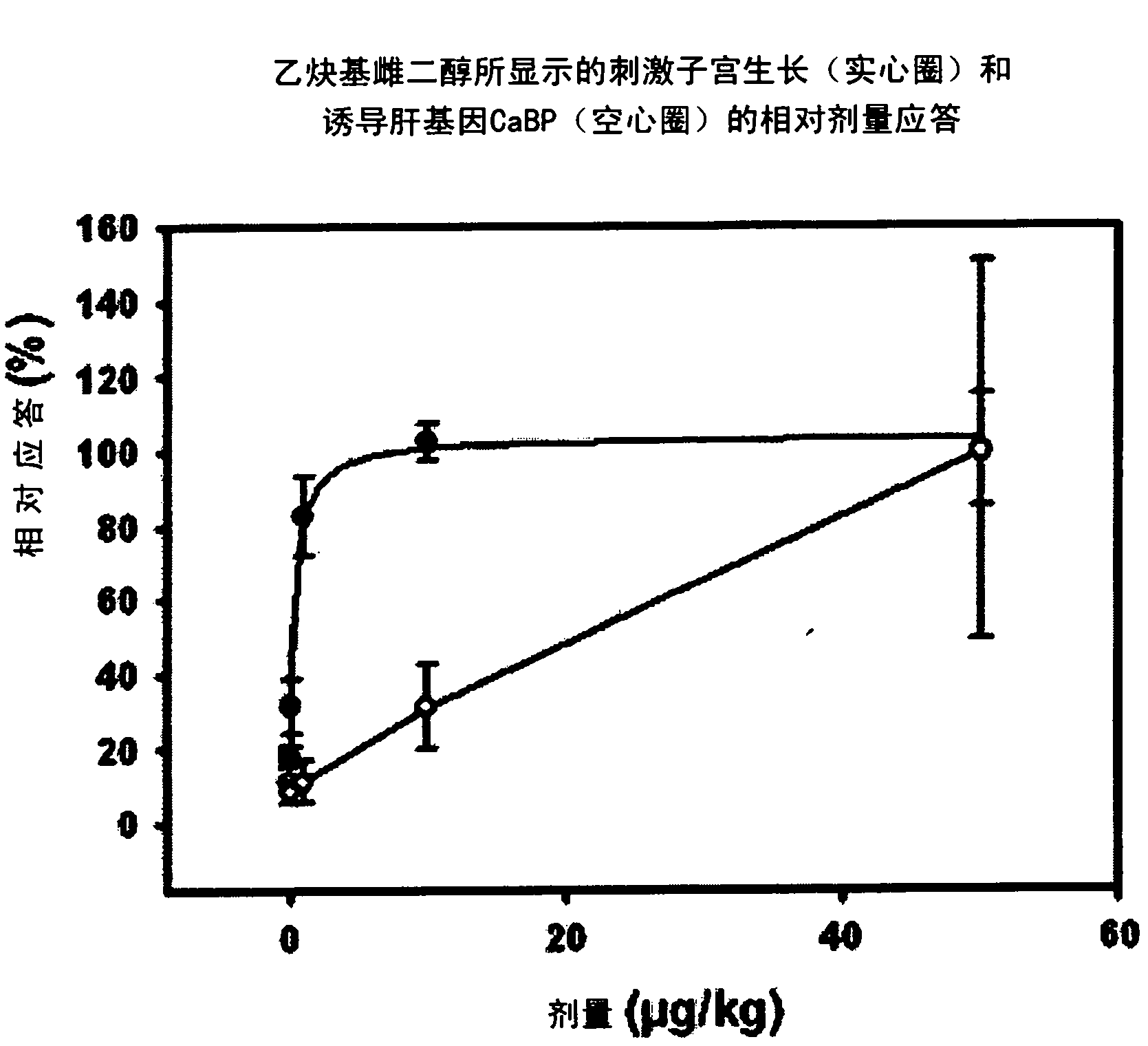
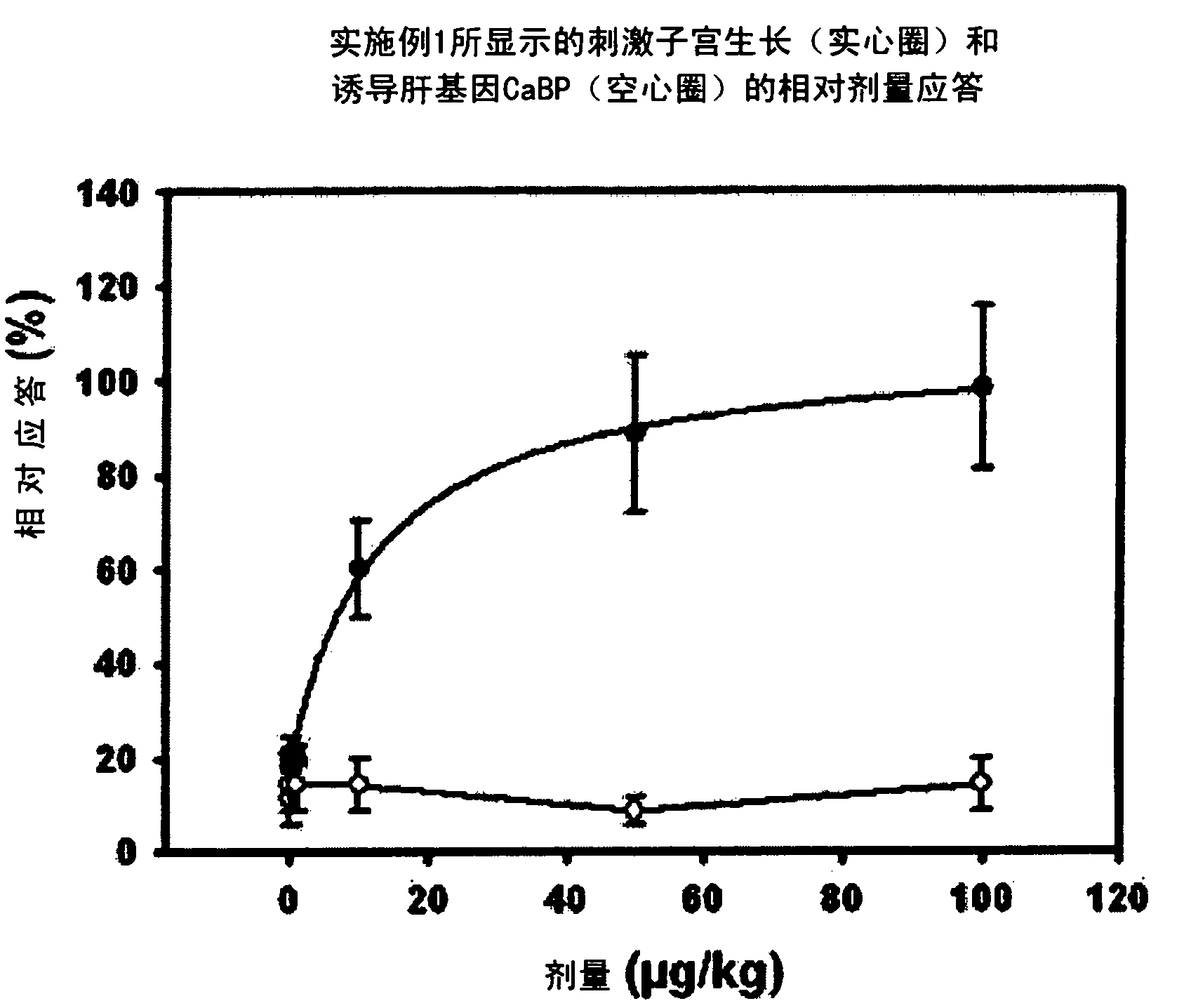
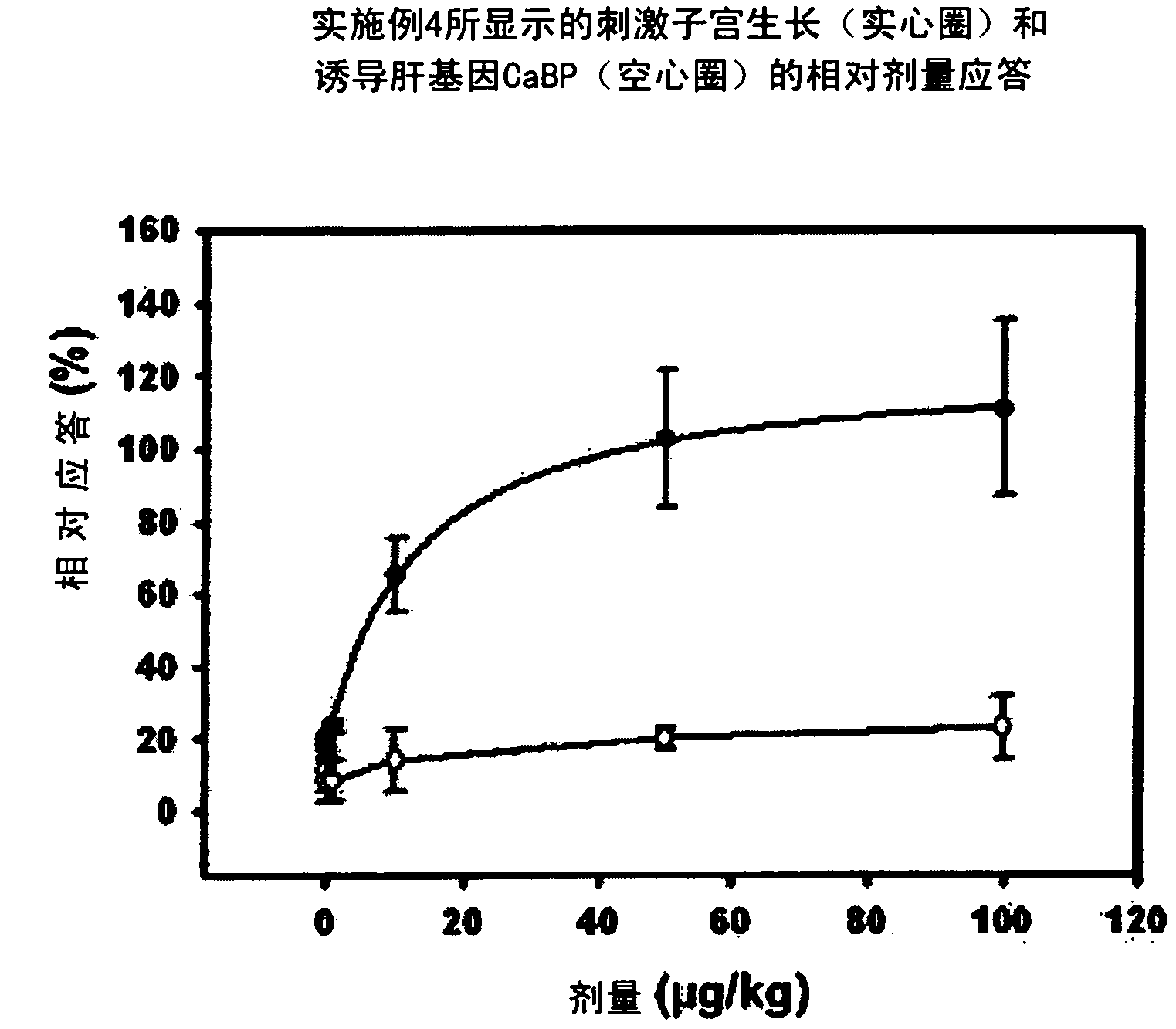
Estratriene derivatives comprising heterocyclic bioisosteres for the phenolic a-ring
An alkenyl and compound technology, applied in the field of estriene derivatives of heterocyclic bioisosteres containing phenol A ring, can solve the problems of increased risk of venous thromboembolism and the like, and achieve the effect of reducing hepatic estrogen activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0184] 2'H-pyrazolo[3',4':3,4]estra-1,3,5(10)-trien-17β-ol
[0185] a) 17β-hydroxy-4(Z)-hydroxymethylene-5α-estr-1-en-3-one
[0186]
[0187] A solution of 500 mg of 17β-hydroxy-5α-estr-1-en-3-one in 10 ml of pyridine and 15 ml of ethyl formate was cooled to -10°C. Sodium methoxide solution (15ml, 1M) was added portionwise. The mixture was allowed to warm to room temperature over two hours. The reaction mixture was poured into ice water and neutralized with hydrochloric acid, extracted with ethyl acetate, dried over sodium sulfate and concentrated under vacuum to give 540 mg of a yellow oil. This material was used in the next step without further purification and characterization.
[0188] b) 2'H-pyrazolo[5',4':3,4]-5α-est-1-en-17β-ol
[0189]
[0190] To a solution of 520 mg crude 17β-hydroxy-4(Z)-hydroxymethylene-5α-estr-1-en-3-one in 6 ml ethanol was added a solution of hydrazine in THF (1.9 ml, 1M). The mixture was stirred at room temperature for 3 hours. The m...
Embodiment 2
[0199] 2'H-pyrazolo[3',4':3,4]estra-1,3,5(10)-trien-17-one
[0200]
[0201] To 521mg 2'H-pyrazolo[3',4':3,4]estra-1,3,5(10)-triene-17β-alcohol (Example 1) in 19ml dichloromethane and 0.7ml To the suspension in pyridine was added 746 mg of Dess-Martin oxidizer. The reaction mixture was stirred at room temperature for 2.5 hours. The reaction mixture was diluted with water and extracted with ethyl acetate. The combined organic extracts were dried over sodium sulfate and concentrated under vacuum. The crude product was triturated with dichloromethane and hexanes to give 325 mg of 2'H-pyrazolo[3',4':3,4]estra-1,3,5(10)-triene-17 as a white solid -ketone.
[0202] MS(CI+): m / z=295(M+H) + ;
[0203] 1 H-NMR (400MHz, CDCl3): δ=8.05 (s, 1H); 7.39 (d, 1H); 7.32 (d, 1H); 3.22 (m, 2H); 2.50 (m, 3H); m, 4H); 1.45-1.80(m, 5H); 0.94(s, 3H); 0.88(t, 1H)
Embodiment 3
[0205] 17α-Methyl-2'H-pyrazolo[3',4':3,4]estra-1,3,5(10)-trien-17β-ol
[0206]
[0207] To a suspension of 80mg 2'H-pyrazolo[3',4':3,4]estra-1,3,5(10)-triene-17-one in 10ml THF was added 2.7ml THF Methylmagnesium bromide (3M). The reaction mixture was stirred for 20 hours. Water was then added, and the mixture was extracted with ethyl acetate. The combined organic extracts were dried over sodium sulfate and concentrated under vacuum. The crude product was chromatographed on silica gel 60 with hexane / ethyl acetate to give 53 mg of 17α-methyl-2'H-pyrazolo[3',4':3,4]estr-1 as a white solid, 3,5(10)-Trien-17β-ol.
[0208] MS(CI+): m / z=311(M+H) + ;
[0209] 1 H-NMR (400MHz, CDCl3): δ=8.03(s, 1H); 7.39(d, 1H); 7.30(d, 1H); 3.16(m, 2H); 2.40(m, 2H); 1H); 2.01(m, 2H); 1.20-1.80(m, 8H); 1.30(s, 3H); 0.92(s, 3H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com