DAA-pyridine as peripheral benzodiazepine receptor ligand for diagnostic imaging and pharmaceutical treatment
A pharmaceutical, phenyl-based technology in the field of DAA-pyridine as a peripheral benzodiazepine receptor ligand for diagnostic imaging and drug therapy, which can solve the problem of low signal-to-noise ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
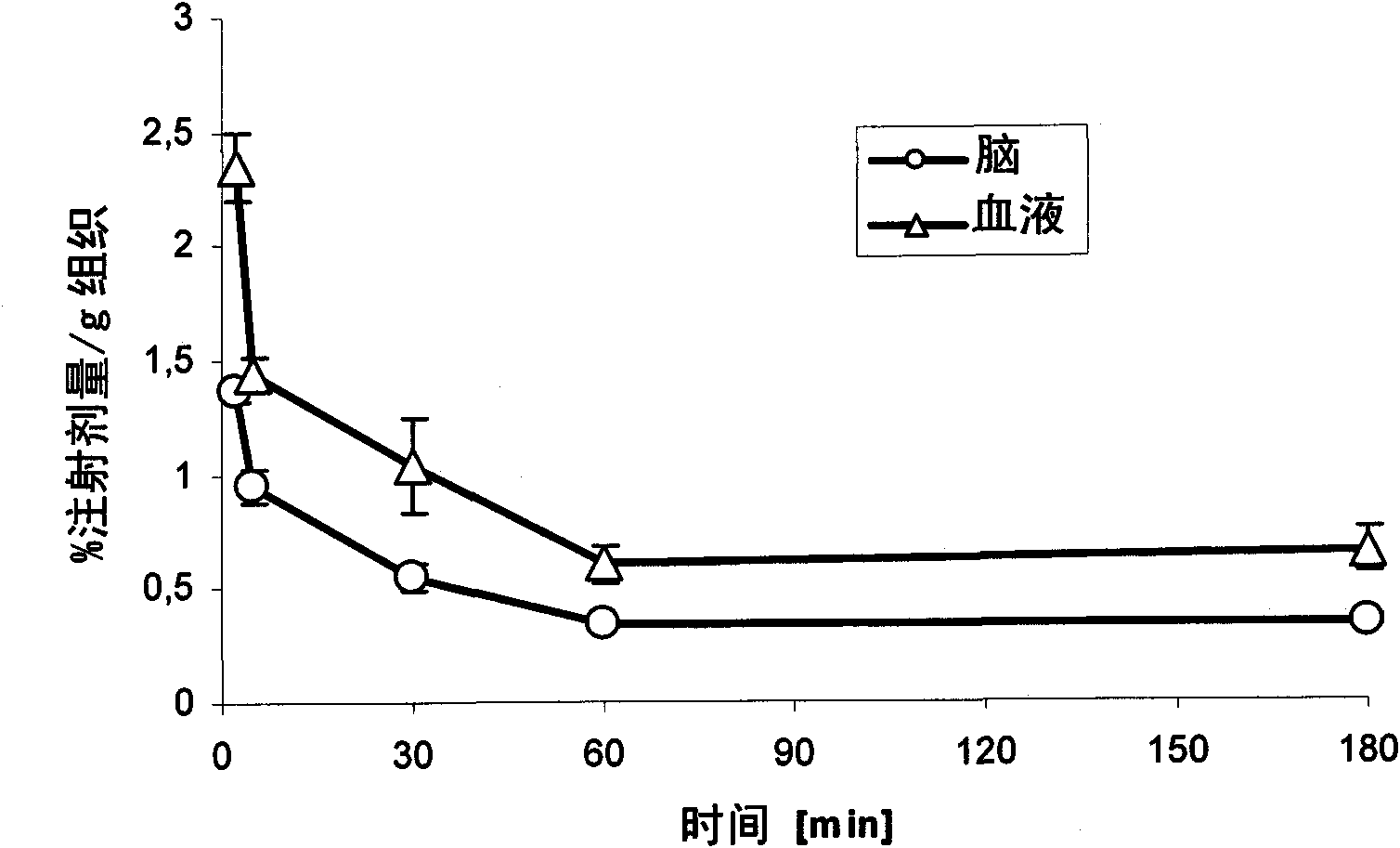
Examples
no. 1 approach
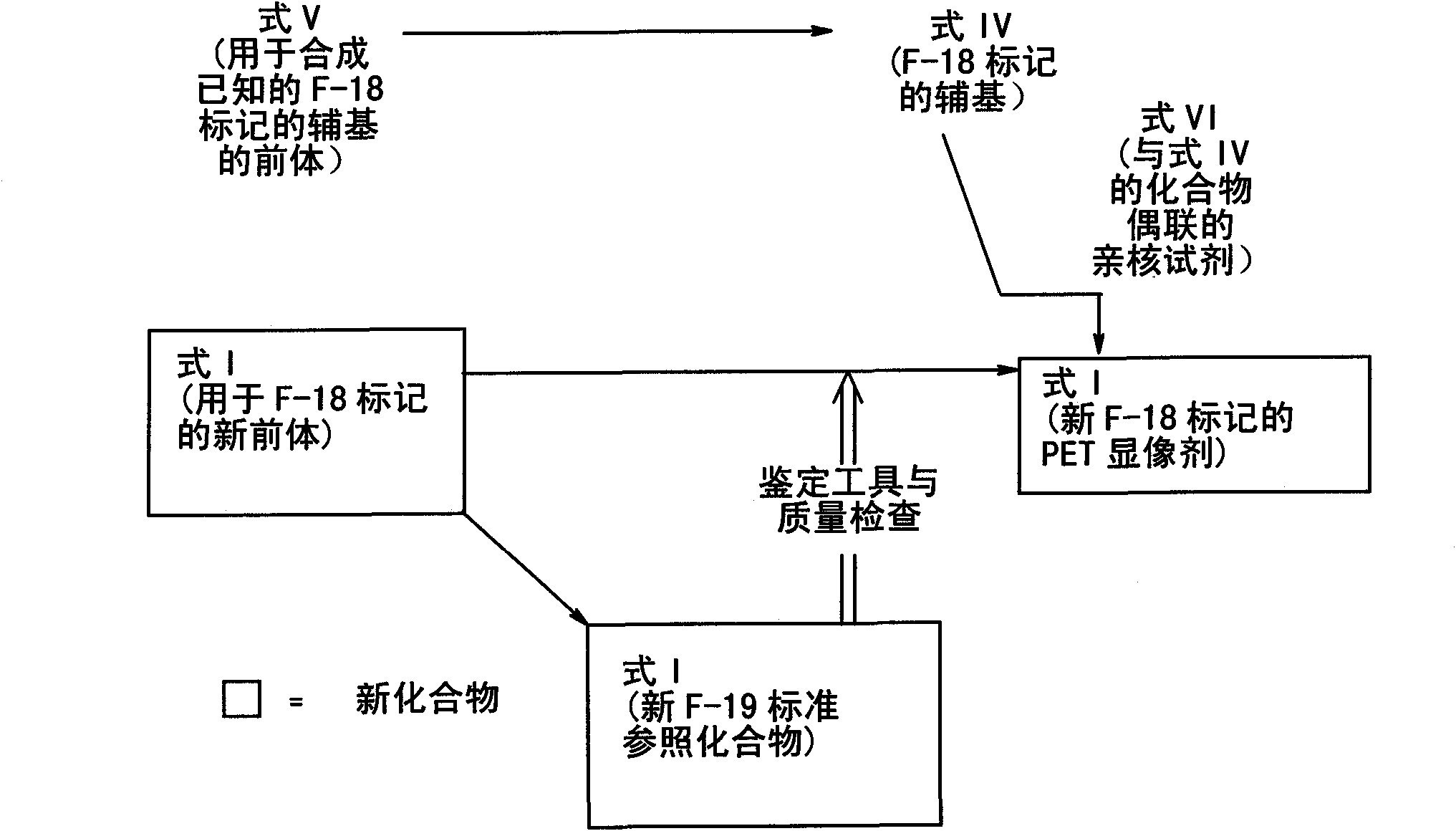
[0364] More specifically, for 18 F-marked formula I compound for obtaining 18 A first embodiment of the radiolabeling method of the F-labeled compound of formula I comprises the following steps:
[0365] - subjecting a compound of formula I with a suitable leaving group to a fluorinating agent 18 F radiolabeled to obtain 18 F labeled compound of formula I.
[0366] The term "radiolabeled" molecule as used herein generally refers to the 18 F-atoms are introduced into the molecule.
[0367] The fluorinating agent is as defined above, wherein F= 18 F.
[0368] In a second embodiment, L is [ 18 F] fluorine or [ 19 F] the synthetic method of the formula I compound of fluorine comprises the following steps:
[0369] - fluorinating the compound of formula V with F-fluorinating agent F-, to produce the compound of formula IV,
[0370]
[0371] Formula V
[0372]
[0373]Formula IV
[0374] - replacing said compound of formula IV with a compound of formula VI,
[037...
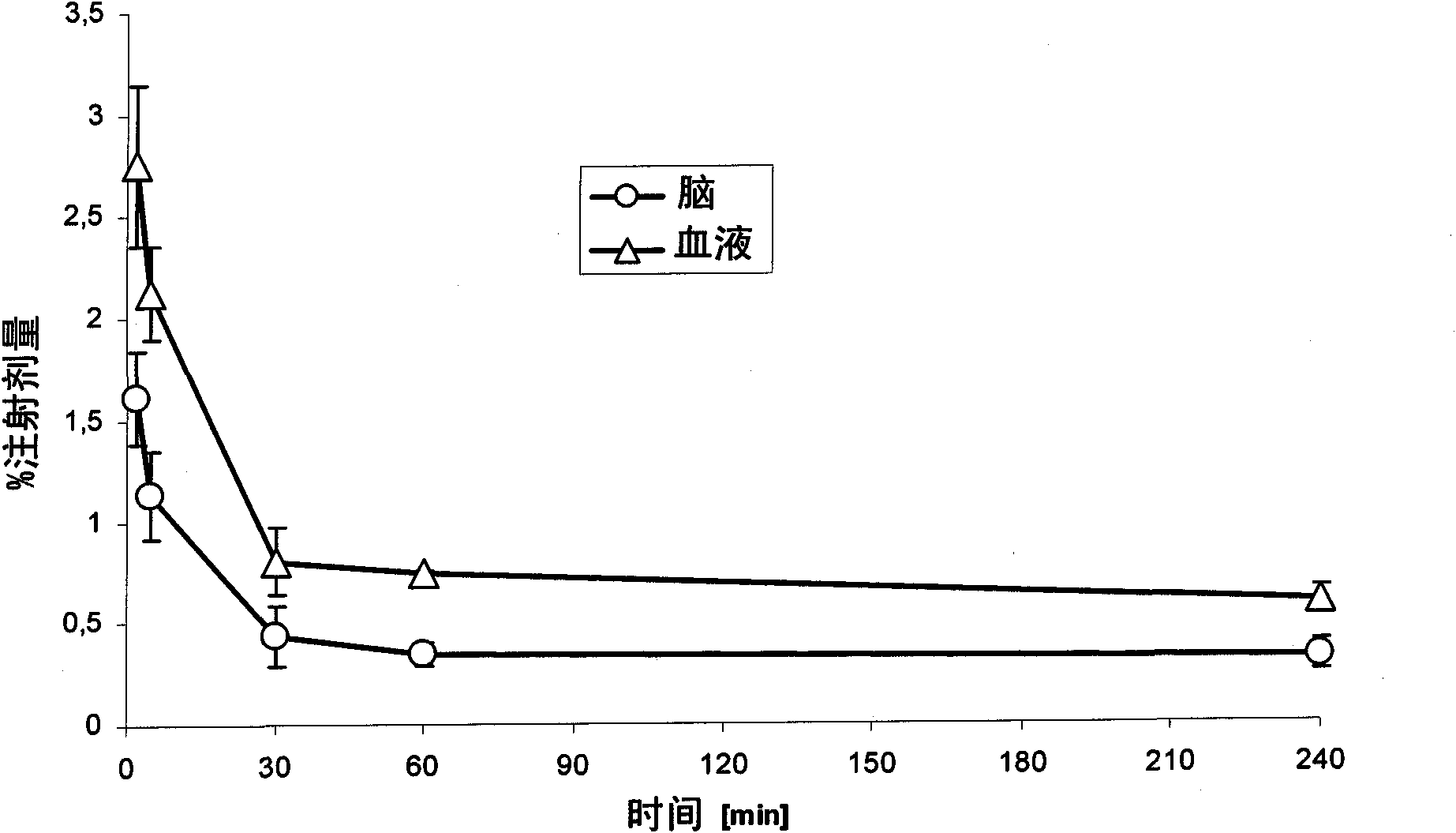
no. 2 approach
[0398] More preferably, for obtaining 18 A second embodiment of the radiolabeling method of the F-labeled compound of formula I comprises the following steps:
[0399] - with fluorinating agent 18 F radiolabels the compound of formula V to produce a compound of formula IV, and
[0400] - Substitution of the compound of formula IV by a compound of formula VI.
[0401] 18 The compound of formula IV marked by F is
[0402]
[0403] or a pharmaceutically acceptable inorganic acid or organic acid salt, hydrate, complex, ester, amide, solvate or prodrug thereof,
[0404] in
[0405] B is a leaving group;
[0406] Leaving group B is known or apparent to those skilled in the art and is derived from, but not limited to, those described or named in the following references: Synthesis (1982), p.85-125, Table 2 (p. 86; (The last item of this Table 2 should be amended to read: "n-C 4 f 9 S(O) 2 -O-nonaflat" instead of "n-C 4 h 9 S(O) 2 -O-nonaflat"), Carey and Sundberg, Org...
PUM
| Property | Measurement | Unit |
|---|---|---|
| radioactivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com