Use of piperlongumine derivatives in preparation of medicines for treating cancers and medicinal compositions thereof
A technology of perylene amide and its derivatives, which can be used in drug combinations, antineoplastic drugs, and pharmaceutical formulations, and can solve problems such as inability to treat normal cells and cancer cells differently, side effects, and normal cell death
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
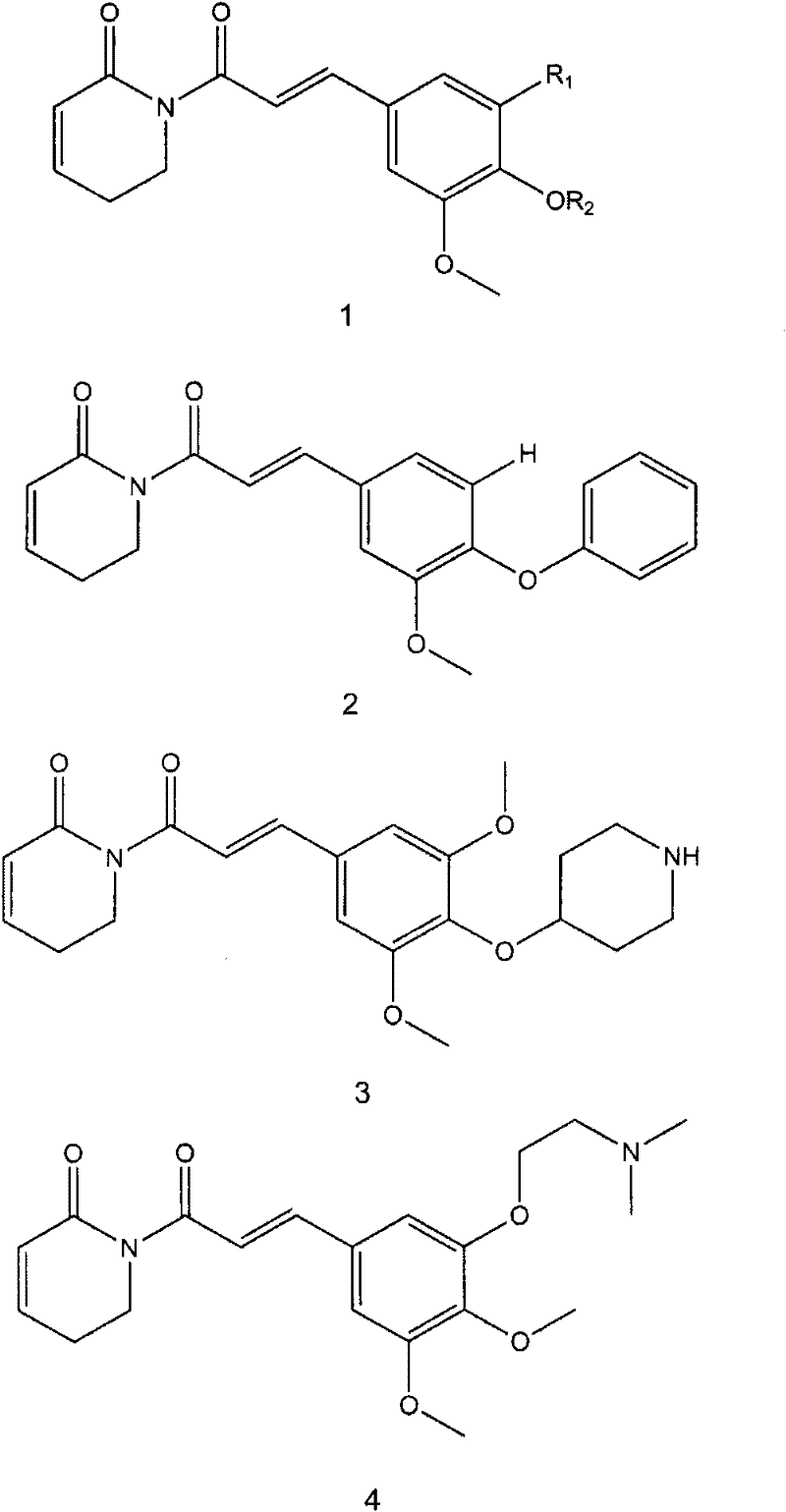
[0037] Compound 1 of the present invention (see attached for the structural formula figure 1 ), when R 1 = -OCH 3 , R 2 =CH 3 The compound a is extracted from natural plants.
Embodiment 2
[0039] Compound formula 2 is synthesized as follows:
[0040]
[0041] The first step: in a 100 mL round bottom flask, add 1 g (1 equivalent) of reactant (1) and 4.10 g (1 equivalent) of reactant (2). Under the protection of argon, add 50 mL of anhydrous DMF solvent. After all the reactants were dissolved, 272 mg (1.1 equivalent) of sodium hydride was added at 0°C. After the reaction was stirred at 0°C for 2 hours, the temperature was gradually raised to room temperature; then the stirring was continued overnight. After the reaction is completed, most of the DMF is removed under high vacuum, and then the reaction concentrate is extracted with dichloromethane. The organic phases were combined, washed with saturated sodium bicarbonate and saturated brine, and concentrated with a rotary evaporator. Finally, the reaction product (3) is purified through a silica gel separation column. Final product: 2.65 g; Yield: 90%. Mass spectrum (HRMS, ESI): 288.1161 (M+1). Elemental analysi...
Embodiment 3
[0045] Compound formula 3 is synthesized as follows:
[0046]
[0047] The first step: In a 100 mL round-bottom flask, add 1 g (1 equivalent) of reactant (1) and 4.72 g (1 equivalent) of reactant (5). Under argon protection, add 50 mL of anhydrous DMF solvent. After all the reactants were dissolved, 272 mg (1.1 equivalent) of sodium hydride was added at 0°C. After the reaction was stirred at 0°C for 2 hours, the temperature was gradually raised to room temperature; then the stirring was continued overnight. After the reaction is completed, most of the DMF is removed under high vacuum, and then the reaction concentrate is extracted with dichloromethane. The organic phases were combined, washed with saturated sodium bicarbonate and saturated brine, and concentrated with a rotary evaporator. Finally, the reaction product (6) is purified by a silica gel separation column. Final product: 2.77 g; Yield: 85%. Mass spectrum (HRMS, ESI): 318.1259 (M+1). Elemental analysis: C, 64.35;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com