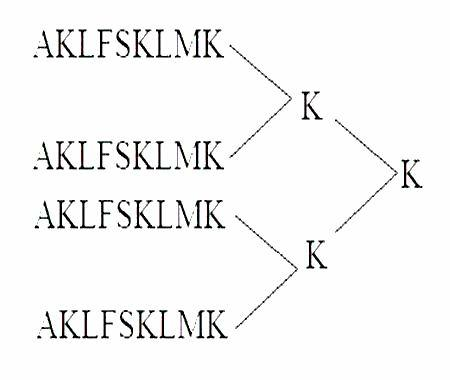
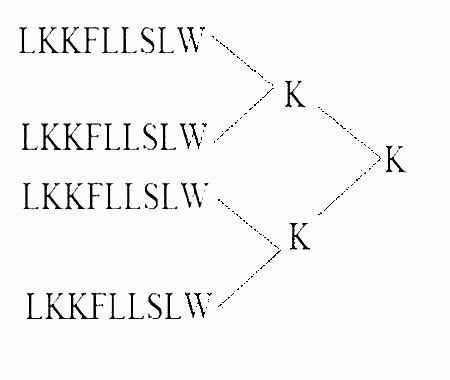
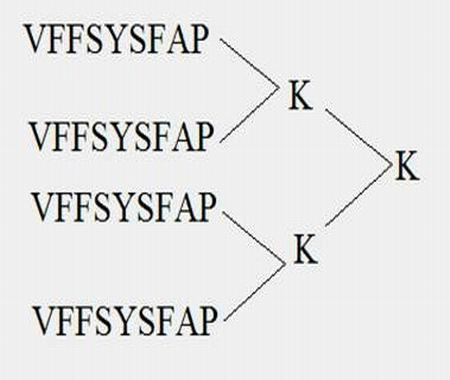
Oncotherapy type heparanase MAP vaccine
A technology of heparinase and peptide vaccine, applied in anti-tumor drugs, antibody medical components, peptide/protein components, etc., can solve the problems of short half-life, inability to induce, small molecular weight, weak antigenicity, etc., and achieve major commercial industrialization value , the effect of increasing molecular mass and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0049] Example 1 Obtaining of the polypeptide epitope MAP vaccine of the present invention
[0050] (1) Synthesis of MAP4 polypeptide
[0051] The peptide synthesis was carried out on the ABI431A peptide synthesizer produced by PE Company in the United States. The brief description is as follows: the standard Fmoc protocol was adopted, and arginine was coupled twice, and extended from the carboxy-terminus to the amino-terminus according to the peptide sequence. After the peptide is synthesized, it is cleaved with the corresponding cleavage enzyme, and the crude peptide products of various protections are removed at the same time. Store at -20°C for later use.
[0052] (2) Purification and molecular weight analysis of heparanase MAP epitope
[0053] Purify and analyze the polypeptide MAP epitope obtained by the above implementation examples by RP-HPLC: dissolve the synthesized MAP4 epitope vaccine with DMSO at a concentration of 20 mg / ml, filter through a 0.22 μm fiber membra...
example 2
[0056] Example 2 Anti-tumor effect research of heparanase MAP4 vaccine of the present invention
[0057] Using standard 4h 51 Cr release method
[0058] ① Preparation of effector cells: DC cells were isolated from PBMC cells of HLA-A2 positive healthy blood donors, and 30 μmol / L heparanase antigen peptide was added to prepare effector cells routinely.
[0059] ② Preparation of target cells: KATO-Ⅲ gastric cancer cells (Hpa + , HLA-A2 + ), U2OS osteosarcoma cells (Hpa + , HLA-A2 + ), SW480 colon cancer cells (Hpa + , HLA-A2 + ) was prepared for routine culture and cryopreservation in our laboratory.
[0060] ③ Detection of CTL activity: according to different effector / target cell ratios, use 51 CTL activity detected by Cr release method
[0061] The specific killing efficiency of effector cells was calculated according to the following formula:
[0062]
[0063] Killing effect of effector cells on target cells Figure 25 .
example 3
[0064] Example 3 Study on Heparanase Specificity of Anti-tumor Heparanase MAP4 Vaccine of the Present Invention
[0065] Using standard 4h 51 Cr release method
[0066] ① Effector cell preparation: Same as above
[0067] ② Target cell preparation: MCF-7 breast cancer cells (Hpa - , HLA-A2 + ), MCF-7 breast cancer cells transfected with heparanase gene (MCF-7 / Hpa) were prepared by this research institute. HepG2 liver cancer cells (Hpa + ,HLA-A2.1 ?? ), HepG2 liver cancer cells transfected with HLA-A2.1 gene (HepG2 / HLA-A2.1) (Hpa + , HLA-A2 + ).
[0068] ③ use 51 The Cr release method detects the killing effect of effector cells on heparanase-negative target cells, and then transfects the heparanase-negative target cells with the full-length gene sequence of heparanase, and then detects the killing effect of effector cells on target cells. use 51 The killing effect of effector cells on HLA-A2.1 negative and heparanase positive target cells was detected by Cr release m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com