Humanization tumor immune cell cytokines TNTIL2 as well as preparation method and application thereof
A technology of TNTIL2 and immune cells, applied in chemical instruments and methods, antineoplastic drugs, pharmaceutical formulations, etc., can solve the problems of lack of treatment specificity, toxic and side effects, clearance, etc., to reduce pain and economic burden, tumor specificity High, extended half-life effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Preparation of humanized tumor immune cytokine TNTIL2
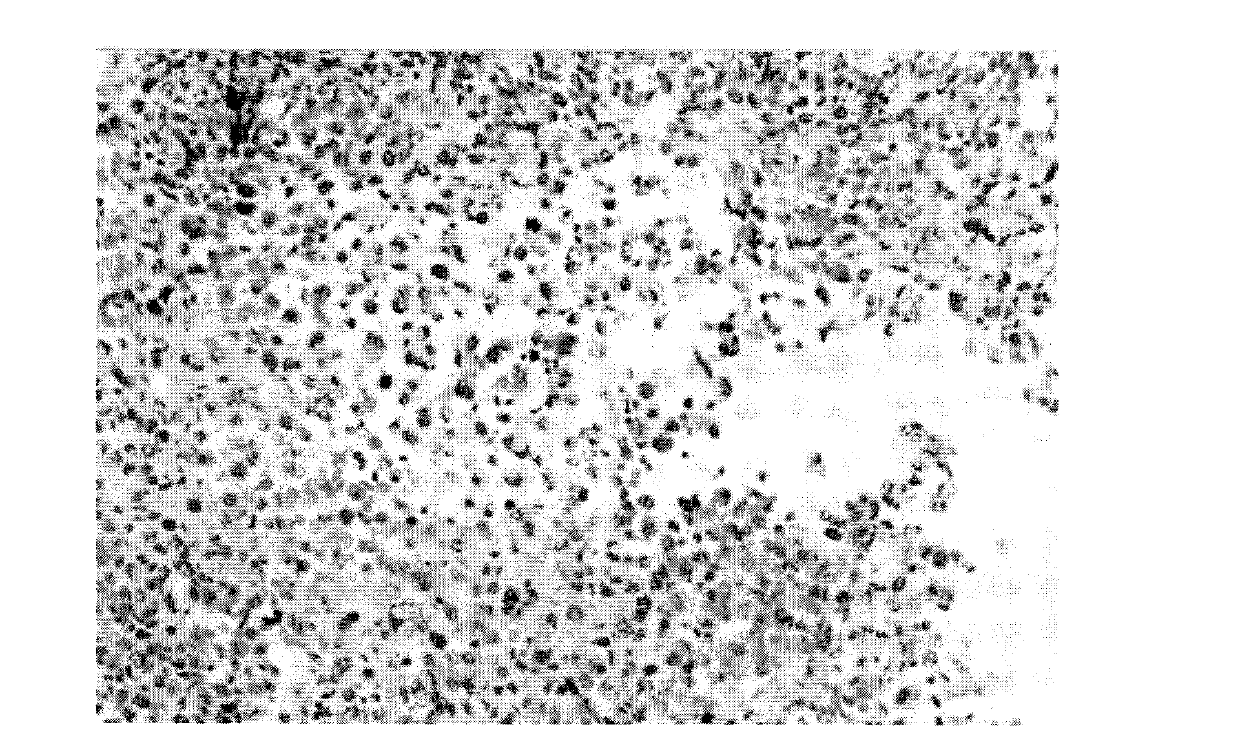
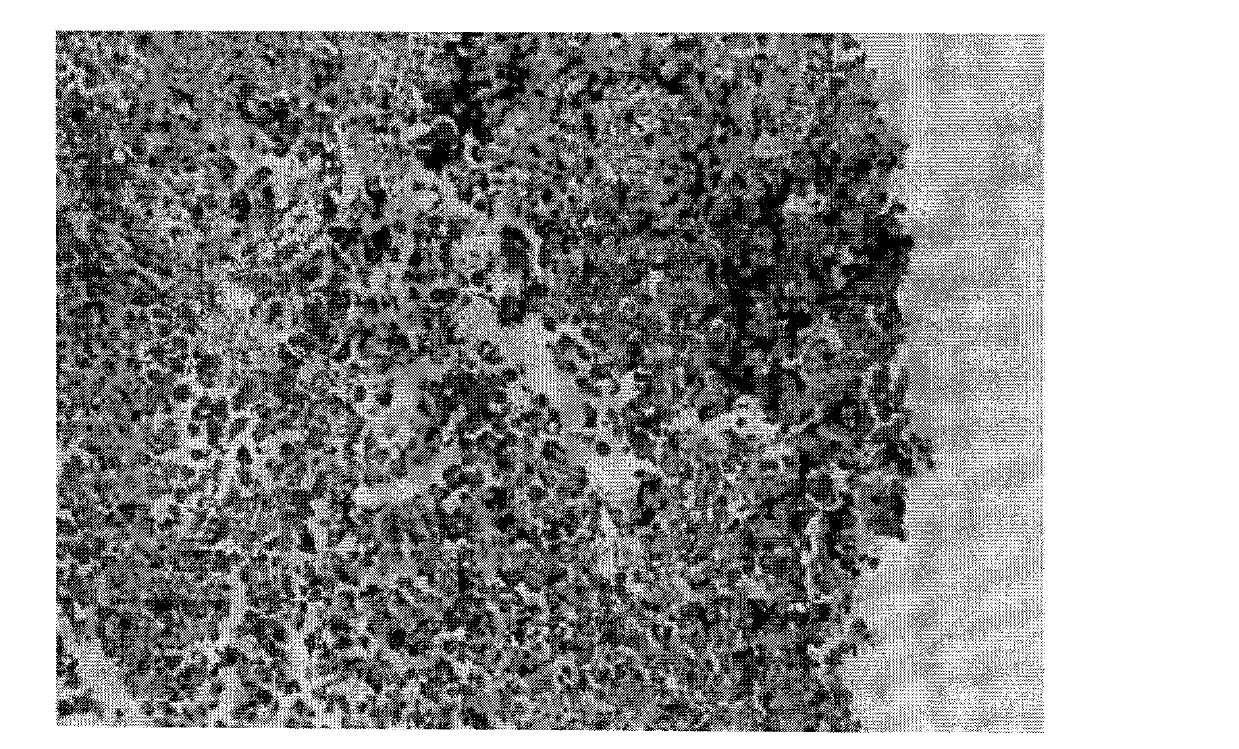
[0040] Using Raji Burkitt's lymphoma cell line (used to produce the immunogen of the hybridoma), the cells were subjected to nuclear extraction. Eight-week-old Balb / c mice were immunized with nuclear extracts. Four days after the last immunization, mice were sacrificed and spleens were removed under aseptic conditions. The spleen was processed into a single cell suspension, and mixed with 8-azaguanine-resistant murine myeloma NS-1 in 40% polyethylene glycol 1540 to construct fusion cells. The mixed cells were then cultured in 96-well tissue cell plates in RPMI-1640 medium containing hypoxanthine, aminopterin, and thymidine. The mouse-derived TNT monoclonal antibody (abbreviation: muTNT-MAb) fusion cell secretory strain positive for nuclear immunofluorescence reaction of fixed Raji lymphoma cells was selected and stored in liquid nitrogen.
[0041] Use PCR and RT-PCR amplification techniques to obtain c...
Embodiment 2
[0048] Example 2 Preparation and quality standard of humanized immune cytokine TNTIL2
[0049] The preparation process is: NS0 shake flask cell culture, bioreactor cell culture, cell supernatant collection, supernatant pretreatment before purification, Protein A affinity purification, SP-Sepharose cation exchange purification, Q-Sepharose anion exchange purification, sterile filtration , stored at 20°C.
[0050] verification of reference products,
[0051] In accordance with the technical procedures for the production and verification of the original solution, the identification of the original solution is carried out. The main test results are shown in Table 2.
[0052] Table 2 TNT-IL2 stock solution identification and measurement project results
[0053] Test items
Embodiment 3
[0054] Example 3 Preparation of powder injection containing immune cytokine TNTIL2
[0055] Using the conventional freeze-drying method, using water as the solvent, including steps: take the fusion protein TNTIL2, add excipients, add water to dissolve, add activated carbon, filter and sterilize, fill, half-stopper, freeze-dry, stopper and cap. Can. The used excipients are selected from one or more of mannitol, hydrolyzed gelatin, glucose, lactose, dextran and the like. Each bottle contains 10-100 mg of fusion protein TNTIL2, which is stored at -20°C until use.
[0056] Or adopt the spray drying method to prepare the powder injection, using water as the solvent, including the steps: take the fusion protein TNTIL2, add or not add excipients (excipients are the same as above), add water to dissolve, add activated carbon, filter and sterilize, spray dry, without Pack the bacteria separately, and just press the plug and roll the cap. Each bottle contains 10-100 mg of fusion prot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com