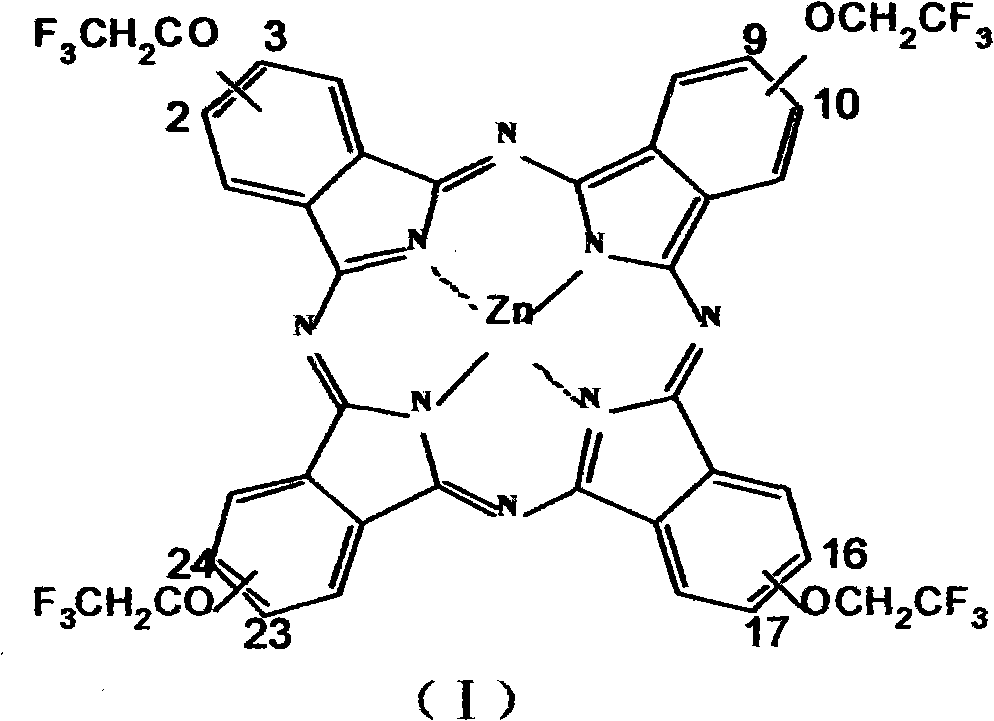
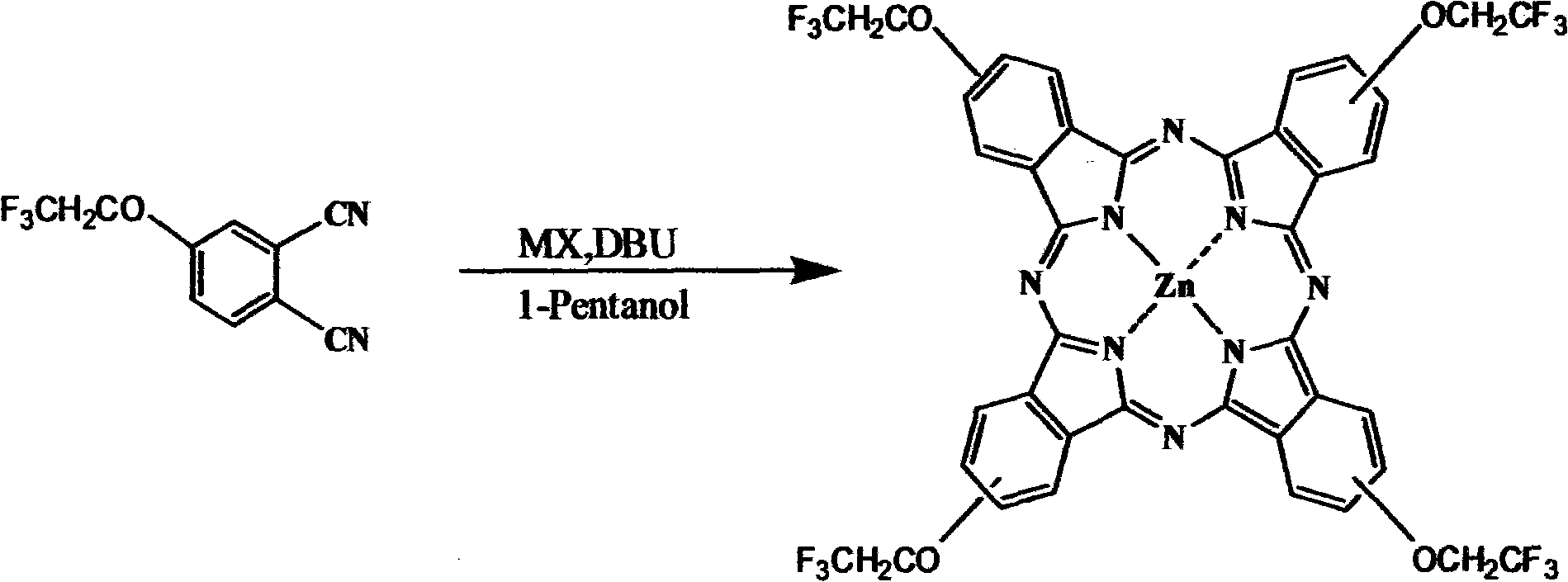
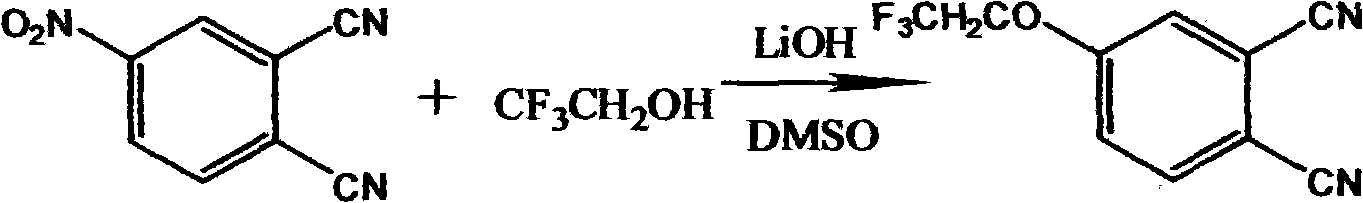Medical photosensitizer and preparation method thereof
A technology of photosensitizer and photodynamic therapy, which is applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, wave energy or particle radiation treatment materials, etc. It can solve the problem of weakening photodynamic killing of tumor cells and poor stability of metal phthalocyanine photosensitizers , Intermolecular easy polymerization and other problems, to achieve the effect of less isomers, easy purification and separation, and good product solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Synthesis of 4-trifluoroethaneoxyphthalonitrile
[0035]
[0036] Add 40mmol of 4-nitrophthalonitrile and 240ml of dry dimethyl sulfoxide (DMSO) and 40mmol of trifluoroethanol to CaCl 2 In the three-necked flask of the drying tube, keep 25°C for stirring reaction, add 2.4g of lithium hydroxide in 3 batches within 2h, (in the actual test operation, preferably add 0.8g of lithium hydroxide every 40 minutes). After reacting for 72 hours, it was slowly dropped into deionized water to obtain a turbid liquid, which was put into the refrigerator and taken out after freezing. After standing for a period of time, filter, wash with deionized water until neutral, and filter with suction to obtain a solid. Recrystallization from methanol afforded a purified intermediate of 4-trifluoroethaneoxyphthalonitrile. Yield 81%. Measure the infrared spectrogram of 4-trifluoroethaneoxy phthalonitrile, IR (cm -1 ): 3075(Ar, H), 2230(C≡H), 1920, 1590, 1485, 1450, 1420, 1400(C-F), 132...
Embodiment 2
[0047] (1) In vitro photodynamic therapy test
[0048] Preparation of tetrakis-(trifluoroethoxy)zinc phthalocyanine (ZnPcF) emulsion: (a) ZnPcF (67.85 mg) was first dissolved in ethanol (2 mL). The ethanol solution was slowly added to Pluronic F68 (Pluronic F68, F68) aqueous solution (10%, 50mL) while vigorously stirring, and the concentration of ZnPcF in the final solution was 1.3mg·mL -1 (1.34mmol·L -1 ), the concentration of F68 was 9.6% (w / v). (b) At the same time, 2 mL of ethanol was added to F68 aqueous solution (50 mL) as a solvent control solution. The above solution was sterilized by filtration through a 0.45 μm filter membrane and stored in a refrigerator in the dark. F68 refers to Pronic F68.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com