Preparation of mizolastine intermediate
A methylaminopiperidine and reaction technology, applied in the field of industrial synthesis of 4-methylaminopiperidine, can solve the problems of complex process, low yield, and many waste water, and achieve the effects of simplified process, high yield and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
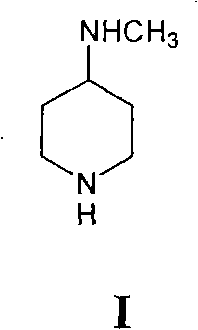
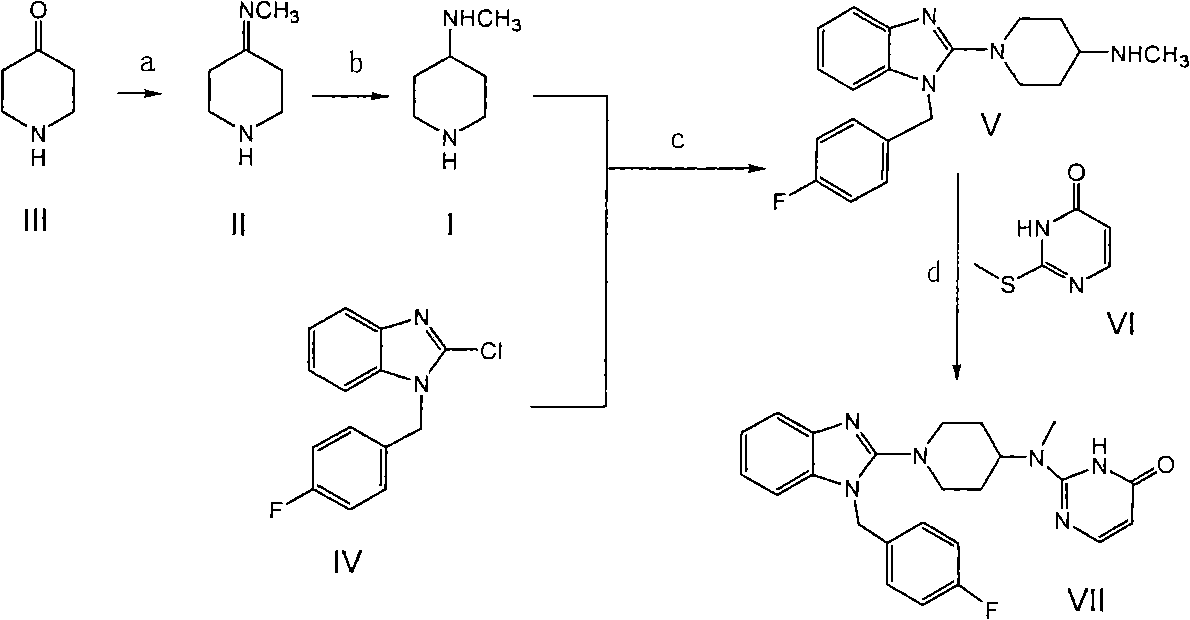
[0015] Embodiment 1 prepares 4-methylaminopiperidine
[0016] Methylammonia-methanol solution: 65Kg of anhydrous methanol is added to the kettle, the temperature is lowered to below 20°C, and 3Kg of methylammonia is passed through to obtain a 0.05% methylammonia-methanol solution. Add 70Kg of methylamine-methanol solution to a 100L enamel reaction kettle, add 10Kg of piperidone hydrochloride, and stir at 20-25°C for 10-12 hours. Transfer to a high-pressure reactor, add 5.42L of 30% ammonia water, 0.8Kg of Raney nickel, a pressure of 1Mpa, a temperature of 25-30°C, and a stirring speed of 400 rpm. The reaction was stirred for 2 hours, and the plate was sampled to determine the end point of the reaction. The pressure is released and the material is discharged. The methanol is first evaporated under normal pressure and then under reduced pressure to obtain 5.0-5.1Kg (5-6.5Kg) of the product.
Embodiment 2
[0017] Example 2 Preparation of 1-{1-(4-fluorophenyl)methyl-1H-benzimidazol-2-yl}-N-methyl-4-piperidinamine
[0018] Put methamphetamine, flubenzimidazole and water into the reaction kettle, control the temperature at 120°C, stir and react for 10 hours, and monitor the reaction by spotting the plate. After the reaction is complete, cool down, filter, evaporate to dryness to remove water, add 12Kg 36% hydrochloric acid, stir for one hour and filter, the filtrate is extracted with 80Kg chloroform, spot plate, after passing the test, the water layer is adjusted to PH=13 with 20% sodium hydroxide, and a solid is precipitated. Filter, wash the filter cake with water, and dry it in the air. Filter and dry to obtain the product (5-6Kg).
Embodiment 3
[0019] Embodiment 3 prepares mizolastine
[0020] Put piperidinamine and methylthiopyrimidinone into a 3L three-necked reaction flask, reduce the pressure to 1-10mmHg with a vacuum pump, heat in an oil bath to 100°C, stir mechanically for ten hours, cool, knock out, and pulverize to obtain 1.1-1.2Kg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com