Method for degrading polyethylene terephthalate
A technology of polyethylene terephthalate and ethylene phthalate, applied in the field of ester degradation, can solve the problems of strong acid and alkali, equipment corrosion, consumption and the like, and achieve the effect of easing reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Put 1.0g of polyethylene terephthalate into the reaction kettle, and add 3.0g of 1-butyl-3-ethylimidazolium bromide ionic liquid, 5.0g of methanol, 0.015g of zinc acetate, and mix well React at 140°C for 6 hours, cool to room temperature after the reaction, add 5mL of methanol to dilute, then filter to remove undegraded polyethylene terephthalate to obtain filtrate a, add water to filtrate a to precipitate precipitate, filter and separate Obtain filtrate b and precipitate, filtrate b is separated and recovered by vacuum distillation to obtain ethylene glycol and 1-butyl-3-ethylimidazolium bromide ionic liquid, dry the precipitate at 30°C for 24 hours, add chloroform to dissolve, filter to remove impurities The filtrate c was obtained, and the chloroform was distilled off from the filtrate c to obtain dimethyl terephthalate (DMT). The yield of DMT was 82.6%.
Embodiment 2
[0019] Put 1.0g of polyethylene terephthalate into the reaction kettle, and add 2.0g of 1-butyl-3-ethylimidazolium bromide ionic liquid, 4.0g of methanol, 0.015g of zinc acetate, and mix well React at 150°C for 5 hours, cool to room temperature after the reaction, add 10mL of methanol to dilute, then filter to remove undegraded polyethylene terephthalate to obtain filtrate a, add water to filtrate a to precipitate precipitate, filter and separate Obtain filtrate b and precipitate, filtrate b is separated and recovered by vacuum distillation to obtain ethylene glycol and 1-butyl-3-ethylimidazolium bromide ionic liquid, dry the precipitate at 30°C for 24 hours, add chloroform to dissolve, filter to remove impurities The filtrate c was obtained, and the chloroform was distilled off from the filtrate c to obtain dimethyl terephthalate (DMT). The yield of DMT was 81.7%.
Embodiment 3
[0021] Put 1.0g of polyethylene terephthalate into the reaction kettle, and add 1.0g of 1-butyl-3-ethylimidazolium bromide ionic liquid, 3.0g of methanol, and 0.015g of lead acetate in sequence, mix well React at 160°C for 4 hours, cool to room temperature after the reaction, add 8 mL of methanol to dilute, and then filter to remove undegraded polyethylene terephthalate to obtain filtrate a, add water to filtrate a to precipitate precipitate, filter and separate to obtain filtrate b and precipitation, the filtrate b was separated and recovered by distillation under reduced pressure to obtain ethylene glycol and 1-butyl-3-ethylimidazolium bromide ionic liquid, dried the precipitate at 30°C for 24 hours, added chloroform to dissolve, and filtered to remove impurities to obtain the filtrate c. Dimethyl terephthalate (DMT) was obtained after distilling off the chloroform from the filtrate c, and the yield of DMT was 83.8%.
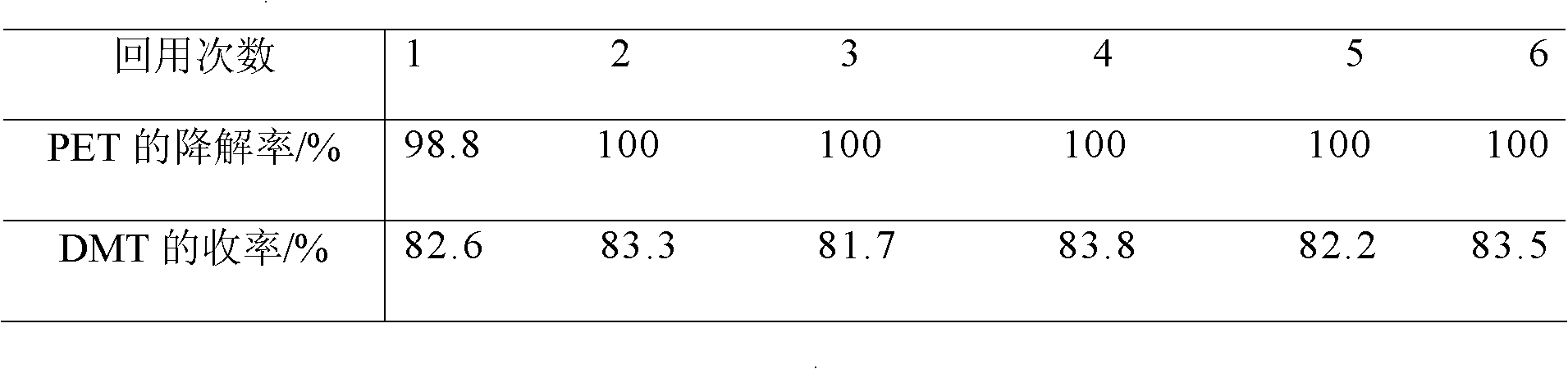
[0022] Table 1 Repeated reuse results of ionic liquids ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com