Structural variants of antibodies for improved therapeutic characteristics
A technology of antibodies, therapeutics, applied in the field of structural variants of antibodies for improved therapeutic characteristics, capable of addressing bone marrow adverse effects, severe infections, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0191] Example 1. Construction of chimeric and humanized anti-CD20 antibodies
[0192] Chimeric (cA20) and humanized (hA20, veltuzumab) anti-CD20 antibodies were constructed as described in U.S. Patent No. 7,151,164, the Examples section of which is incorporated herein by reference (see also, Stein et al., Clin Cancer Res. 2004; 10: 2868-2878). The variable region DNA and amino acid sequence profiles of cA20 and hA20 are disclosed in Figures 1 and 2.
[0193] The CDR sequences of cA20 and hA20 are identical to those of rituximab (parental murine MAb C2B8), except for the third CDR of the heavy chain (CDRH3). For convenience, the heavy chain CDR sequences are referred to as CDRH1-H3 and the light chain CDRs as CDRL1-L3. Of the reported CDRH3 sequences of anti-CD20 antibodies, only C2B8 and the corresponding rituximab have an asparagine residue at Kabat position 101.
[0194] The framework region sequences of veltuzumab (hA20) were constructed using the same human IgG donor f...
Embodiment 2
[0200] Example 2. Construction of the D101N sequence variant of veltuzumab
[0201] All restriction endonucleases and other enzymes were purchased from New England Biolabs (Beverly, MA). Oligonucleotides were synthesized by Sigma Genosys (Haverhill, UK). PCR reactions were performed using Amplitaq polymerase (Applied Biosystems, Foster City, CA) and a Perkin Elmer (Wellesley, MA) GeneAmp PCR system 9600. The hA20-pdHL2 (see U.S. Pat. No. 7,151,164) vector was used as a template and the oligonucleotide primer pair 5' D101N (cggtgactggtacttcaatgtctggggccaaggcaccacg SEQ ID NO: 17) and 3' Hind3 (aaagcttgcggccgcgatcc SEQ ID NO: 18) or 3' D101ccN (cggtgtaggact Two PCR reactions of taccagtcaccg SEQ ID NO: 19) and 5' XhoI (cctcgagcacacaggacctc SEQ ID NO: 20) generated 210 bp or 510 bp amplification primers, respectively. A third PCR reaction using a mixture of 210 bp and 510 bp products as template and 5' XhoI and 3' Hind3 primers generated 680 bp amplification primers, which were g...
Embodiment 3
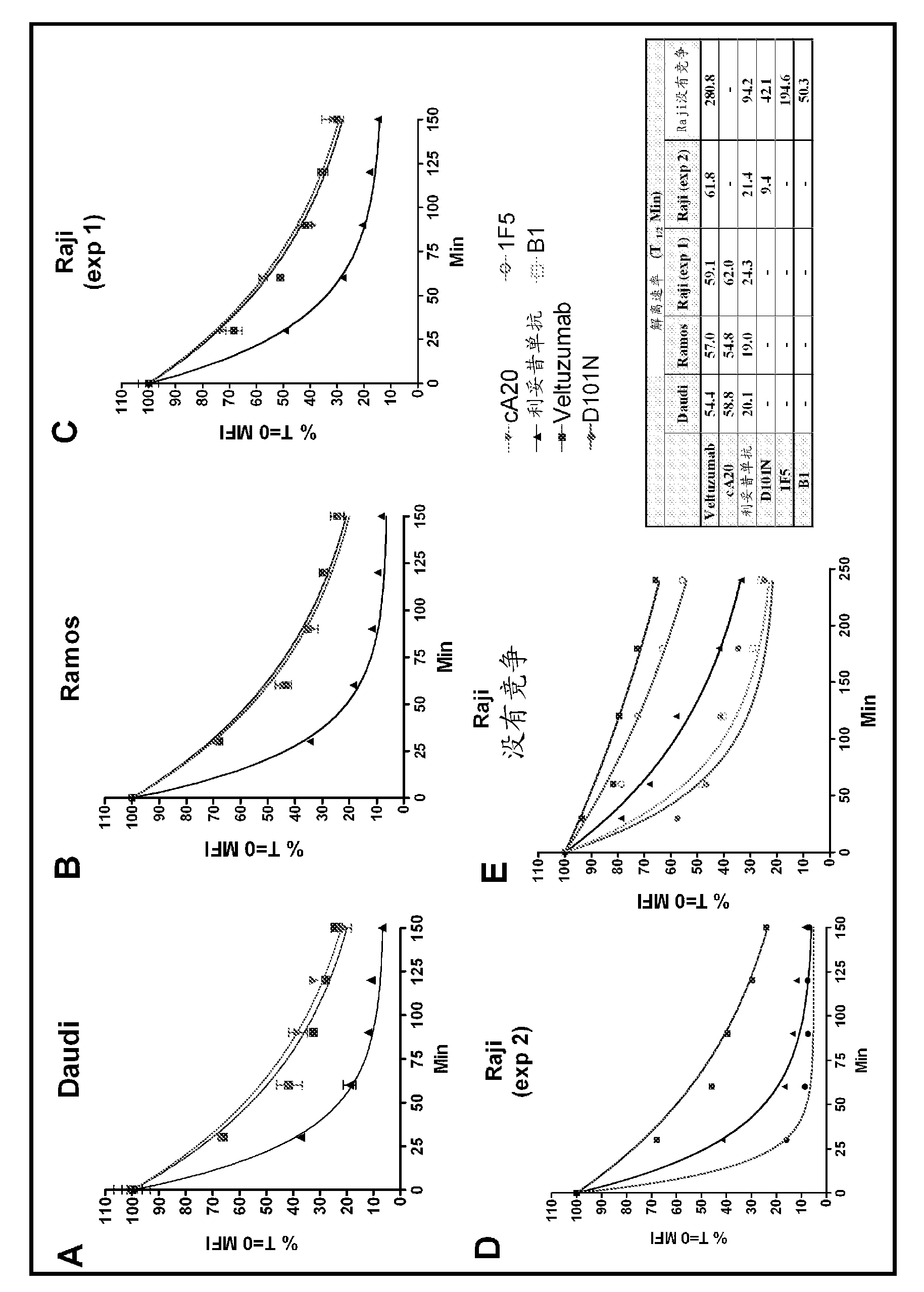
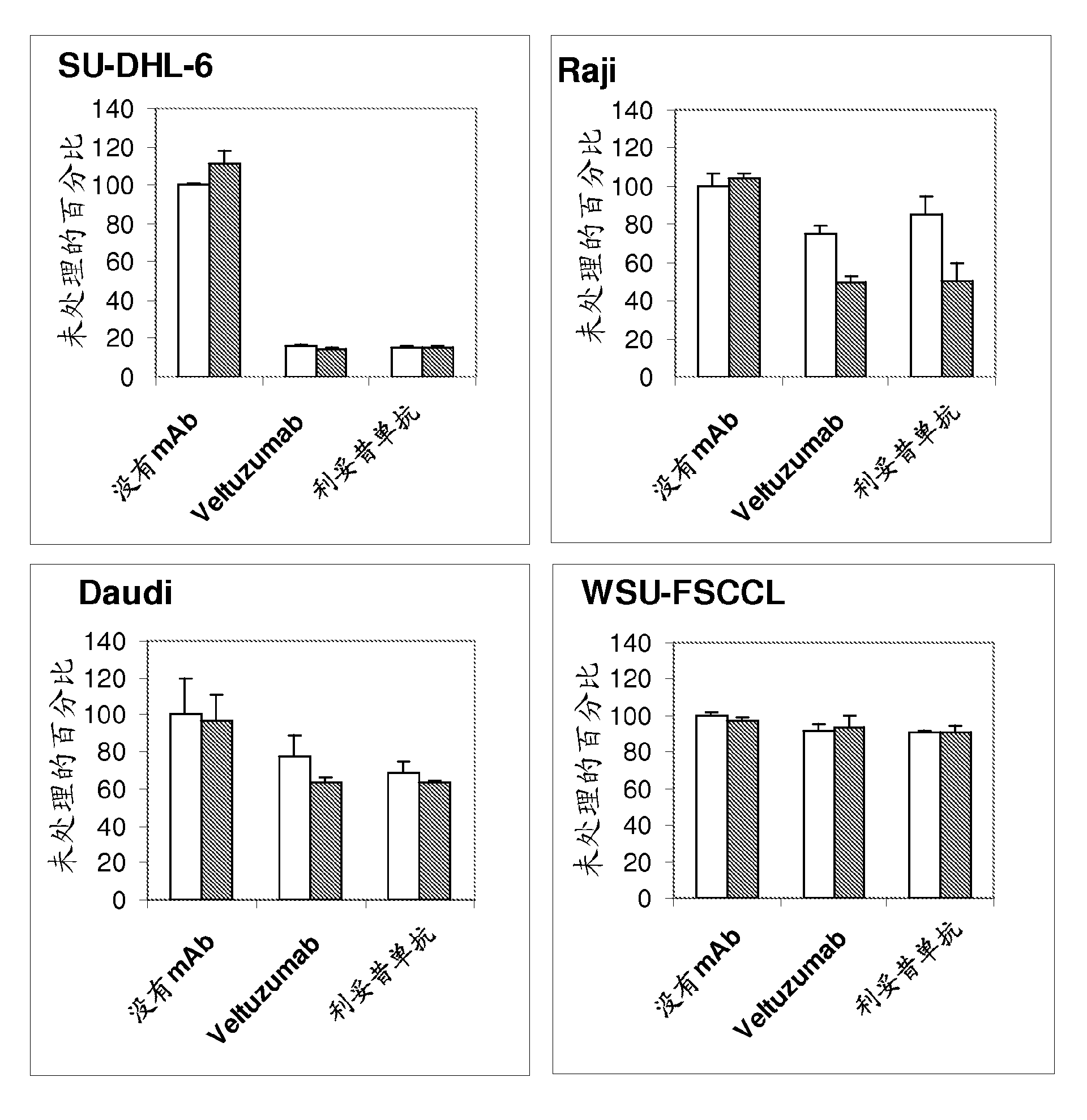
[0202] Example 3. Scatchard Analysis of Binding of Anti-CD20 Antibodies
[0203] cell line
[0204] In the following examples, the murine hybridoma 1F5 and the human Burkitt's lymphoma lines Daudi, Raji and Ramos were purchased from the American Type Culture Collection (Manassas, VA). The non-Burkitt lymphoma cell lines used were: SU-DHL-6 from Dr. Alan Epstein (University of Southern California, Los Angeles, CA) and WSU from Dr. Mitchell Smith (Fox Chase Cancer Center, Philadelphia, PA) -FSCCL. Cultured in DMEM (Life Technologies, Inc. Gaithersburg, MD) supplemented with 10% fetal bovine serum, penicillin (100 units / ml), streptomycin (100 μg / ml) and L-glutamine (2 mM) cells, as a suspension culture.
[0205] Scatchard Analysis
[0206] The maximum number of binding sites per Raji cell and veltuzumab was determined by nonlinear regression analysis of saturated binding data obtained with radioiodinated samples and Raji cells using Prism software (GraphPad Software Inc., San...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com