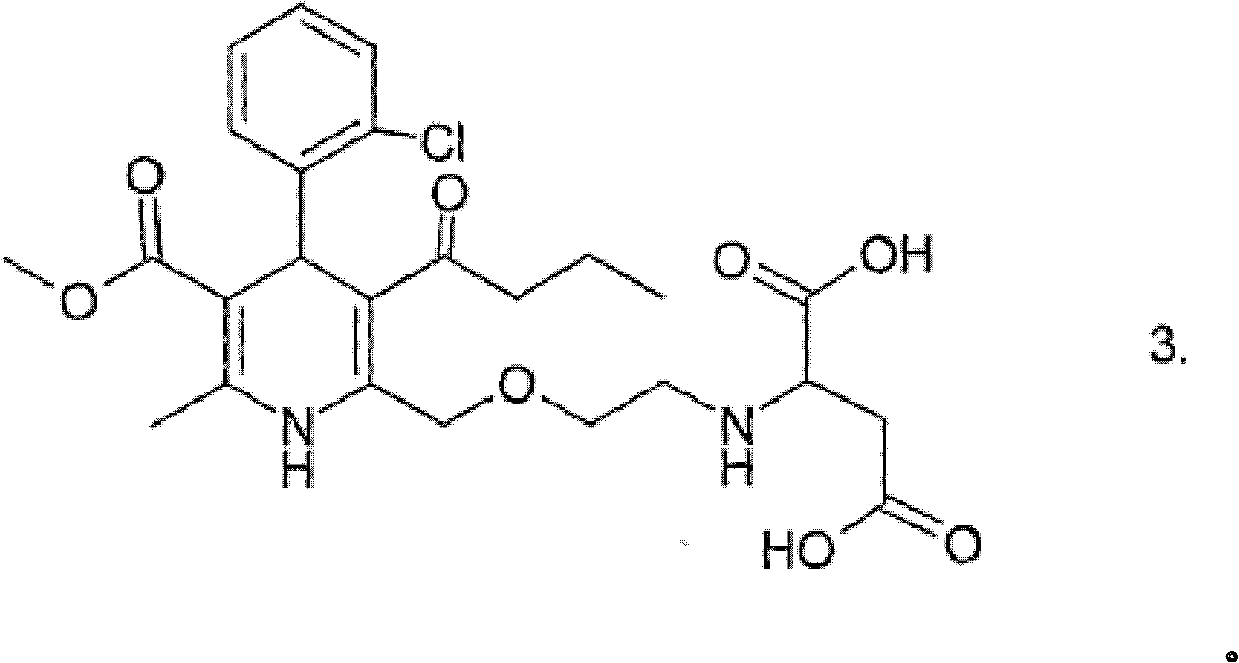
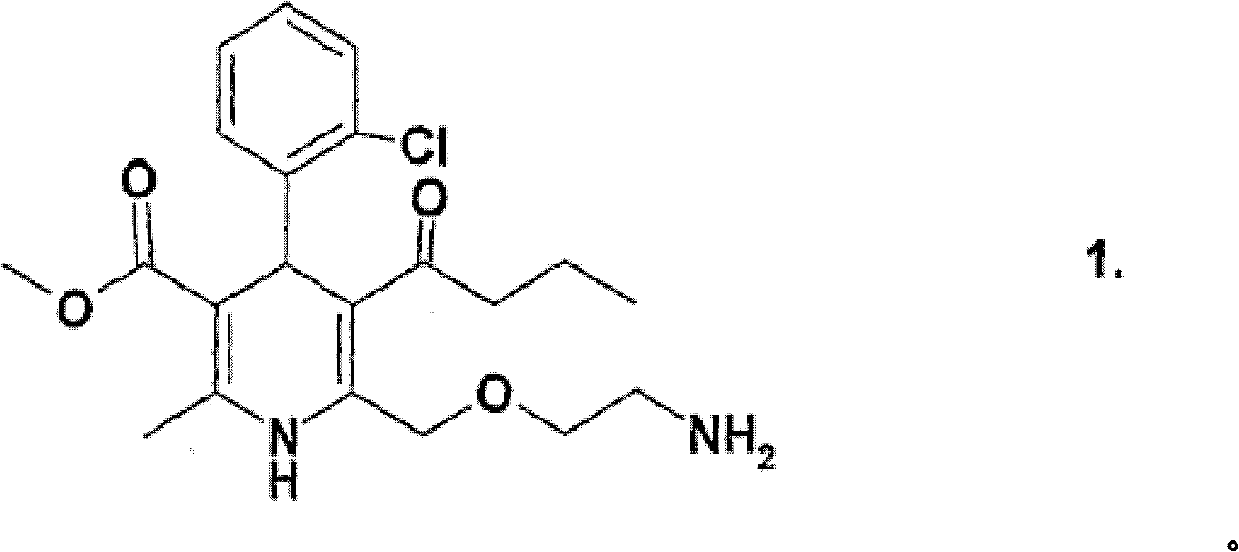
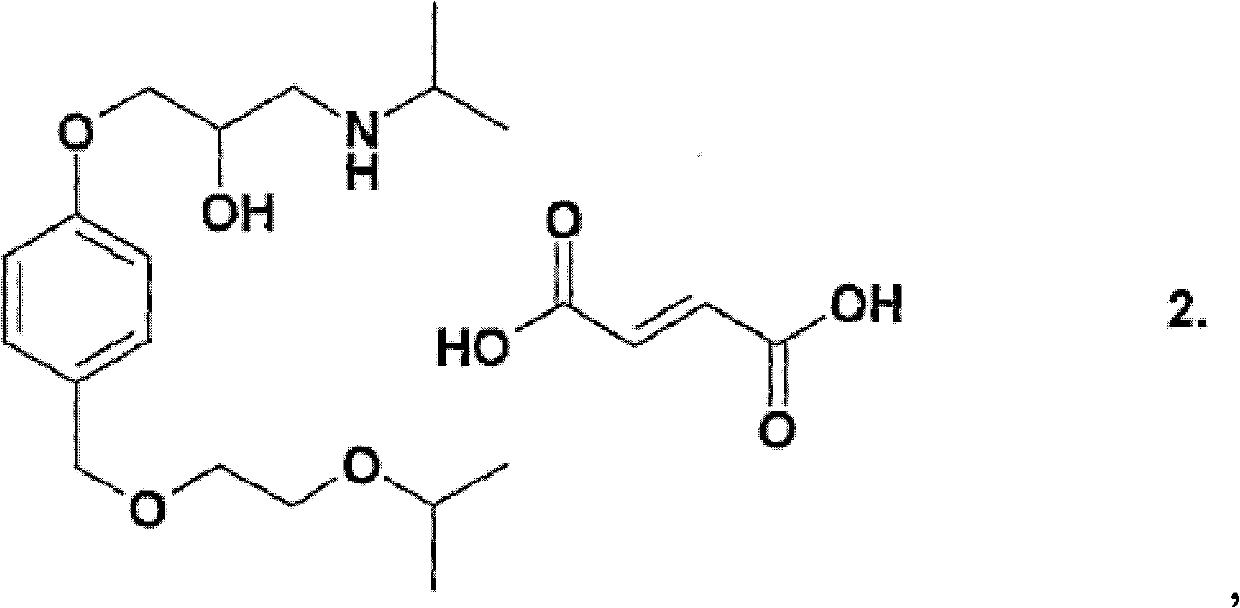
Compositions comrrising amlodipine and bisoprolol
A kind of technology of amlodipine base and amlodipine besylate, applied in the field of solid pharmaceutical compositions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] Composition of 1000 tablets:
[0104]
[0105] method:
[0106] The two active ingredients were sieved through a 250 μm sieve and then homogenized with microcrystalline cellulose, sodium starch glycolate and colloidal silicon dioxide in a drum mixer for 10 minutes. Magnesium stearate was then added to the mixture and the resulting mixture was further homogenized for 2 minutes.
[0107] The homogenate was compressed into tablets having a weight of 305 mg using a tablet machine.
[0108] Tablets were packed into cold-formed OPA / AL / PVC composite foil blisters with a thickness of 130 μm and covered with aluminum foil with a thickness of 20 μm.
[0109] Tablets prepared according to Example 1 were checked for contaminants on the day of manufacture and after storage for 3 months at 40°C / 75% relative humidity as follows:
[0110]
[0111] Further quality data of the product of Example 1 are as follows:
[0112]
Embodiment 2
[0114] Composition of 1000 capsules:
[0115]
[0116] method:
[0117] The two active ingredients were sieved through a 250 μm sieve and then homogenized with microcrystalline cellulose, sodium starch glycolate and colloidal silicon dioxide in a drum mixer for 10 minutes. Magnesium stearate was then added to the powder mixture and the resulting mixture was homogenized for a further 2 minutes.
[0118] The homogenate is filled into capsules with a 47 mg filling using a tablet press.
[0119] Capsules were packed into cold-formed OPA / AL / PVC composite foil blisters with a thickness of 130 μm and covered with aluminum foil with a thickness of 20 μm.
[0120] On the day of production and after storage for 3 months at 40° C. / 75% relative humidity, the results of the contaminant inspection of the capsules prepared and packaged according to Example 2 were as follows:
[0121]
[0122] Reference example:
[0123] Composition of 1000 tablets:
[0124]
[0125] method:
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com