Application of tumor cell membrane selectively penetrating peptide
A tumor cell and penetrating peptide technology, applied in the field of penetrating peptides, can solve problems such as inability to distinguish between ordinary cells and tumor cells, enhanced efficacy and toxic side effects of anti-tumor drugs, and patient suffering.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
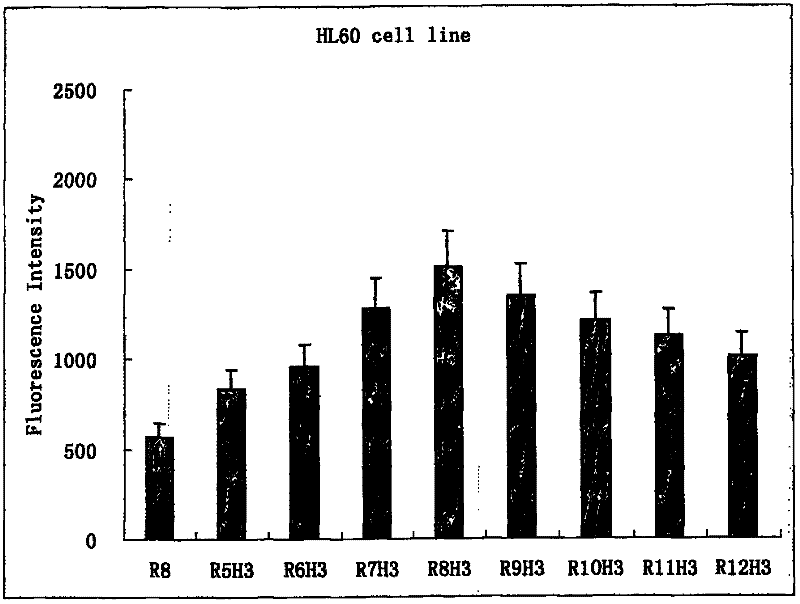
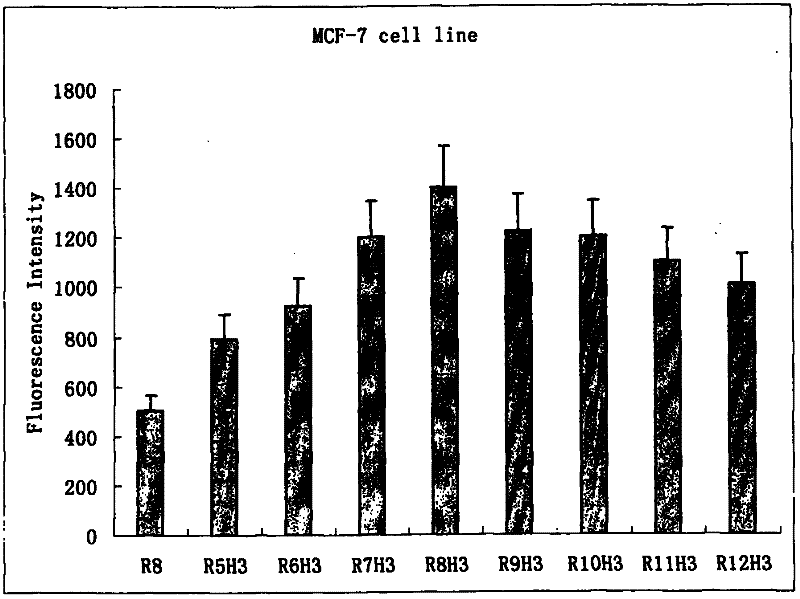
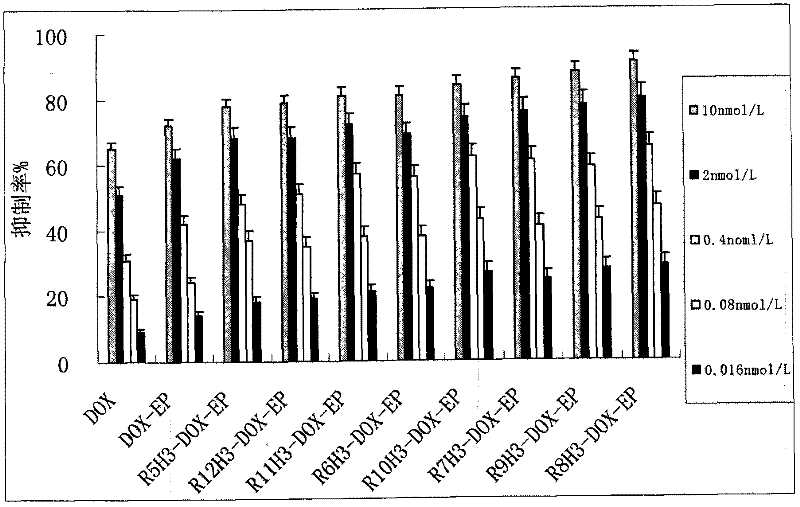
Image
Examples
Embodiment 1
[0018] RRRRRHHH(R 5 h 3 , R at the C-terminus and N-terminus) as an example to illustrate the method of using solid-phase synthesis of polypeptides.
[0019] Add 1g of Rinkamide-MBHA resin (0.74mmol / g) into a 50mL round-bottomed flask with a branch pipe on the side and a sand plate filter element in the branch pipe, add 20mL DMF to swell for 10 minutes, and remove the solvent by suction filtration. Then 20 mL of 20% piperidine / DMF solution was added, stirred for 30 minutes, and suction filtered. The resin was washed 6 times with DMF, and the solvent was removed by suction filtration. Add 1.44g (2.22mmol) Fmoc-Arg(Pbf)-OH (Pbf is 2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl), 0.46g ( 2.22mmol) dicyclohexylcarbodiimide and 0.30g (2.22mmol) HOBt (1-hydroxybenzotriazole) and 20mL DMF, stirring at room temperature for condensation reaction. After 2 hours of reaction, a small amount of resin was taken for ninhydrin color reaction, and the result showed that the condensation...
Embodiment 2
[0023] RRRRRHHH(R 6 h 3 , R at the C-terminus and N-terminus) as an example to illustrate the method of using solid-phase synthesis of polypeptides.
[0024]Add 1.13g of Rinkamide-MBHA resin (0.74mmol / g) into a 50mL round-bottomed flask with a branch pipe on the side and a sand plate filter element in the branch pipe, add 20mL DMF to swell for 10 minutes, and remove the solvent by suction filtration. Then 20 mL of 20% piperidine / DMF solution was added, stirred for 30 minutes, and suction filtered. The resin was washed 6 times with DMF, and the solvent was removed by suction filtration. Add 1.63g (2.51mmol) Fmoc-Arg(Pbf)-OH (Pbf is 2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl), 0.52g ( 2.51mmol) of dicyclohexylcarbodiimide and 0.34g (2.51mmol) of HOBt (1-hydroxybenzotriazole) and 20mL of DMF were stirred at room temperature for condensation reaction. After 2 hours of reaction, a small amount of resin was taken for ninhydrin color reaction, and the result showed that th...
Embodiment 3
[0028] RRRRRRRHHH(R 7 h 3 , R at the C-terminus and N-terminus) as an example to illustrate the method of using solid-phase synthesis of polypeptides.
[0029] Add 1.26g of Rinkamide-MBHA resin (0.74mmol / g) into a 50mL round-bottomed flask with a branch pipe on the side and a sand plate filter element in the branch pipe, add 20mL DMF to swell for 10 minutes, and remove the solvent by suction filtration. Then 20 mL of 20% piperidine / DMF solution was added, stirred for 30 minutes, and suction filtered. The resin was washed 6 times with DMF, and the solvent was removed by suction filtration. Add 1.82g (2.80mmol) Fmoc-Arg(Pbf)-OH (Pbf is 2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl), 0.58g ( 2.80mmol) dicyclohexylcarbodiimide and 0.38g (2.80mmol) HOBt (1-hydroxybenzotriazole) and 20mL DMF, stirring at room temperature for condensation reaction. After 2 hours of reaction, a small amount of resin was taken for ninhydrin color reaction, and the result showed that the condens...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com