New preparation method for Tadalafei crystal form I
A tadalafil, amorphous technology, applied in organic chemistry and other directions, can solve problems such as failure to meet industrialization requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
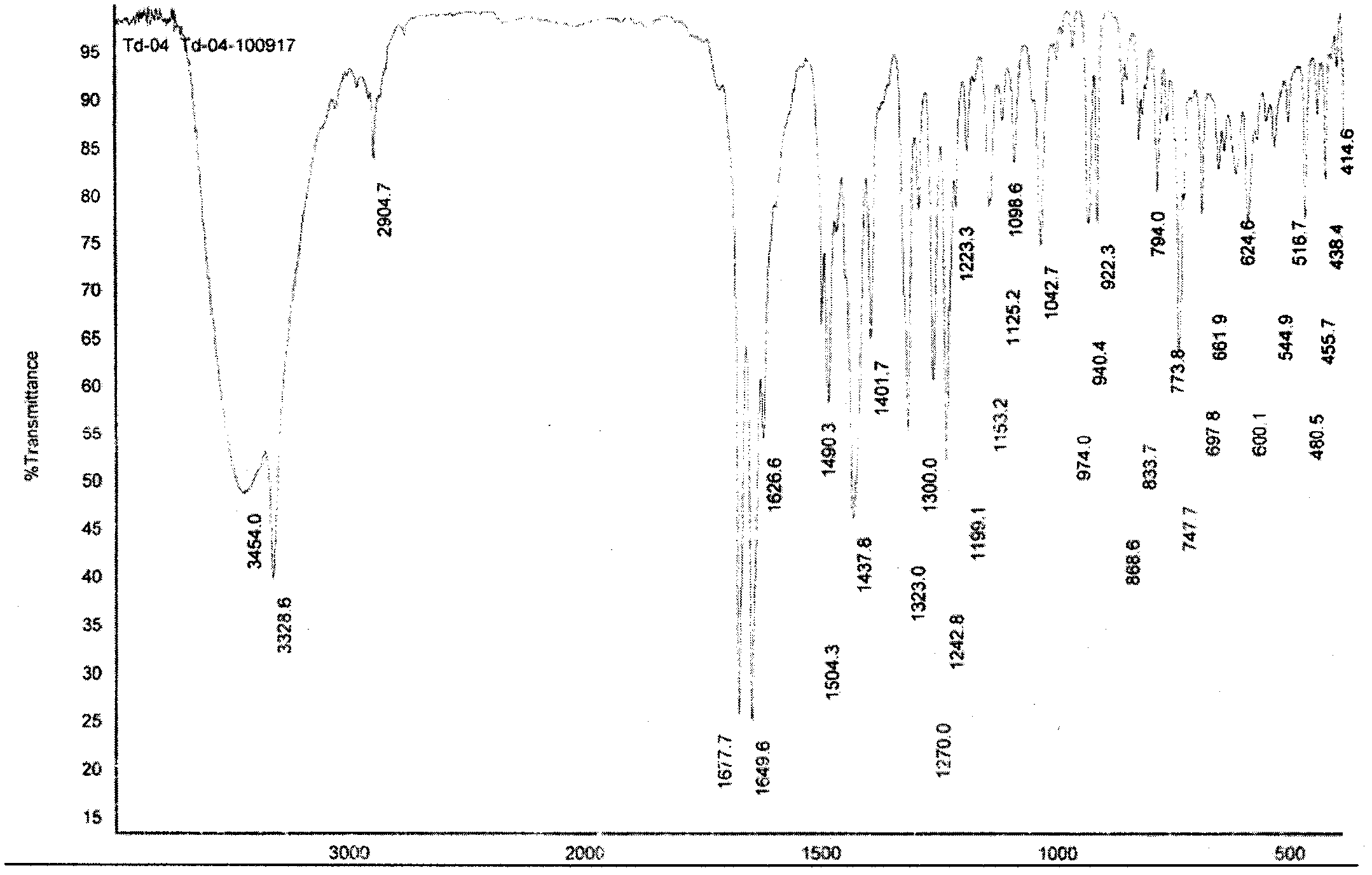
[0048] Add 10.0 g of tadalafil crude product, add 100 ml of 1,3-dioxane, heat to reflux (about 74° C.), and stir until a clear and transparent solution. Slowly lowered to room temperature, stirred for 30 minutes, then cooled to -5°C to 0°C with an ice-salt bath, a large amount of solid was precipitated, kept stirring for 2 hours, filtered, and dried to obtain 8.5 g of tadalafil type I crystals.
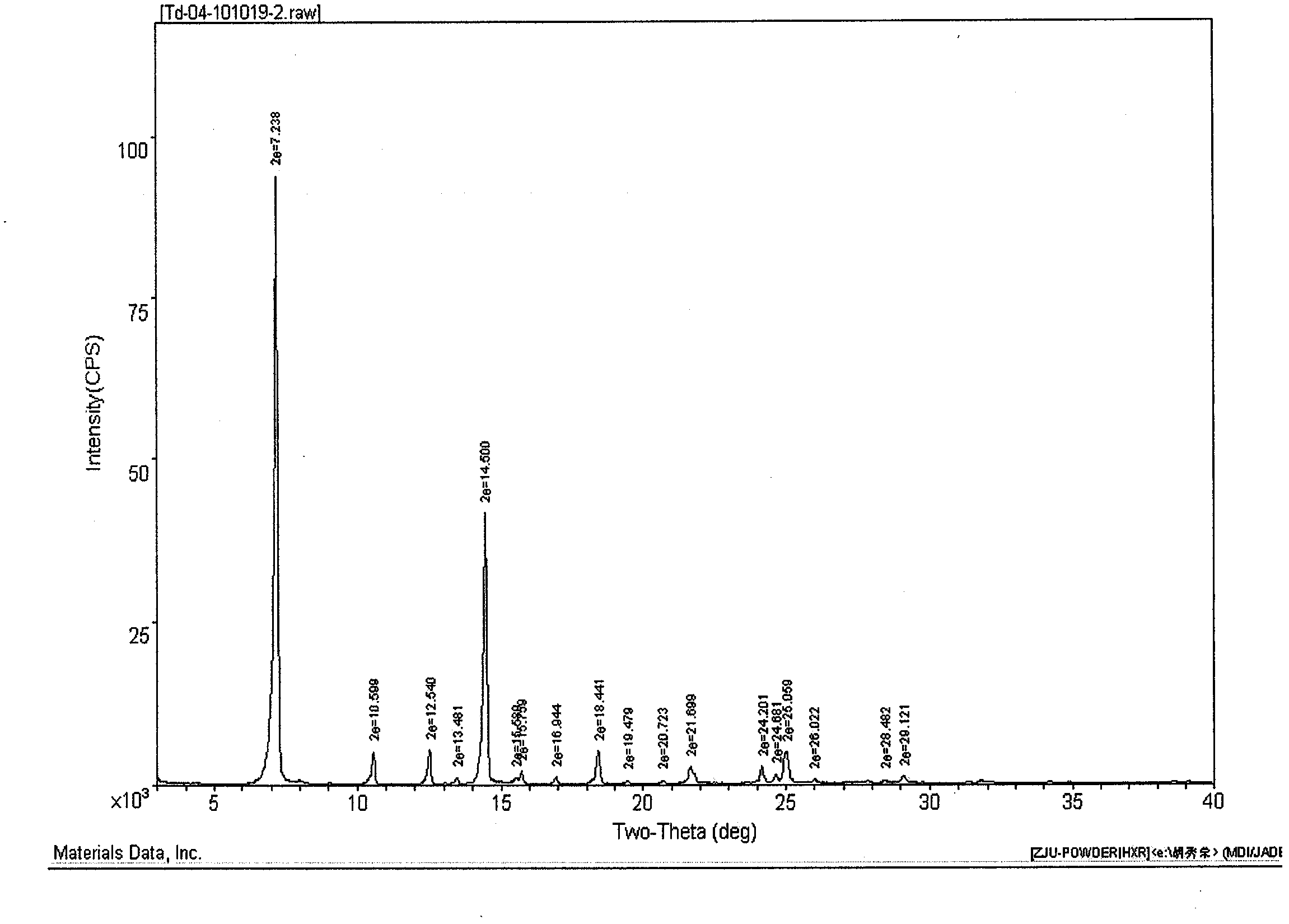
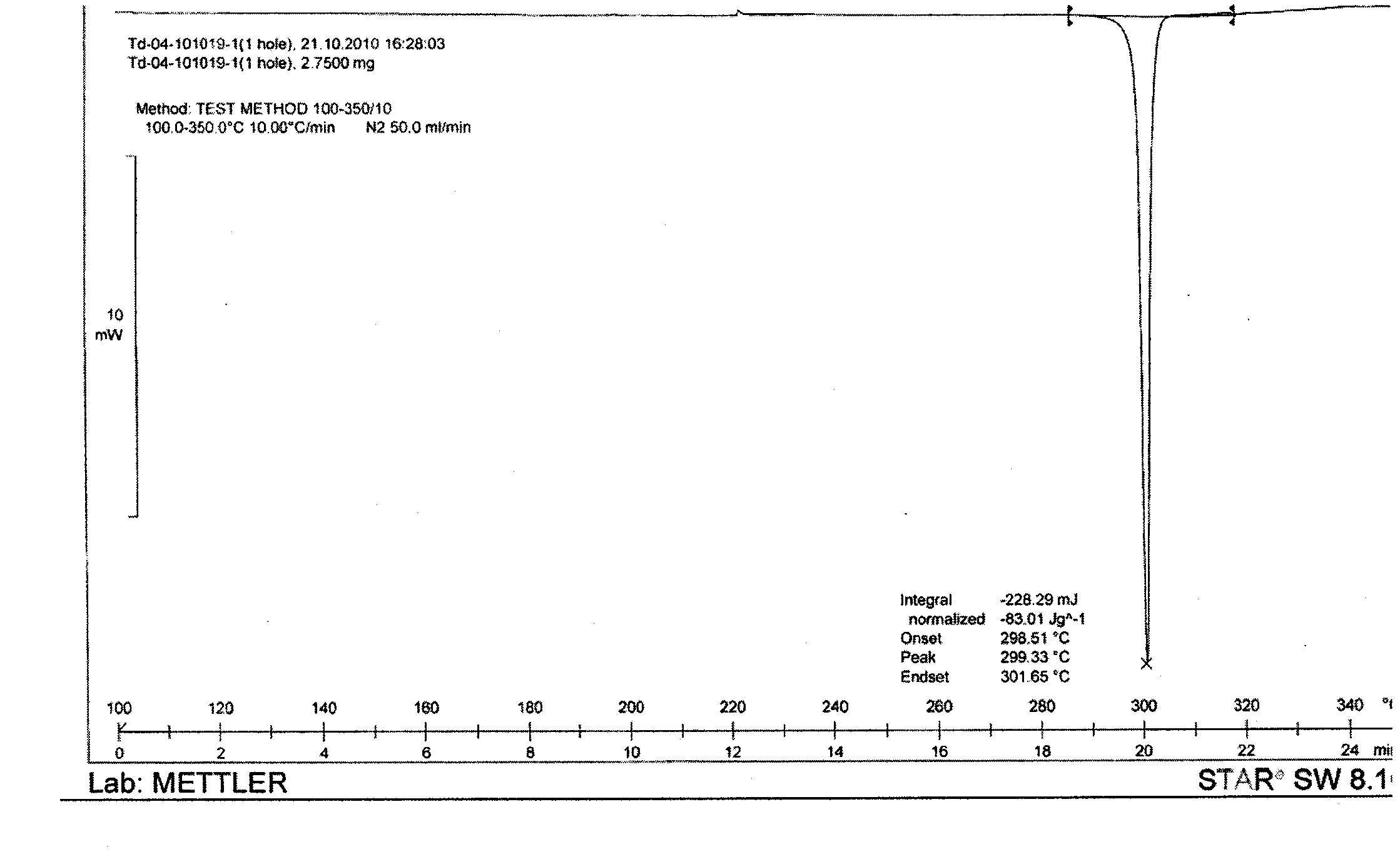
[0049] Yield 85% DSC: 299~302°C HPLC: 99.98%
[0050] Chiral isomer HPLC: 0.02% Trans isomer HPLC: 0.05%
Embodiment 2
[0052] Add 10.0 g of tadalafil crude product, add 50 ml of 1,3-dioxane, heat to reflux (about 74° C.), and stir until a clear and transparent solution. Add 150 ml of water, slowly reduce to room temperature, and then cool to -5 ° C ~ 0 ° C with an ice-salt bath, separate out a large amount of solid, keep stirring for 2 hours, filter, and dry to obtain 9.0 g of tadalafil type I crystals
[0053] Yield 90% DSC: 299.7~303℃
Embodiment 3
[0055] 10.0 g of crude tadalafil was added to 400 ml of ethylene glycol dimethyl ether, heated to dissolve, and stirred to a clear and transparent solution. Slowly lowered to room temperature, then cooled to -5 ° C ~ 0 ° C with an ice-salt bath, a large amount of solid was precipitated, kept stirring for 2 hours, filtered, and dried to obtain 8.0 g of tadalafil type I crystals.
[0056] Yield 80%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com