Production method of epichlorohydrin
A technology of epichlorohydrin and chloropropene, which is applied in sustainable manufacturing/processing, chemical industry, climate sustainability, etc., can solve the problems of low conversion rate and selectivity, difficult control of reaction temperature, etc., and achieve good technology effect, overcome the untimely heat extraction, improve the conversion rate and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
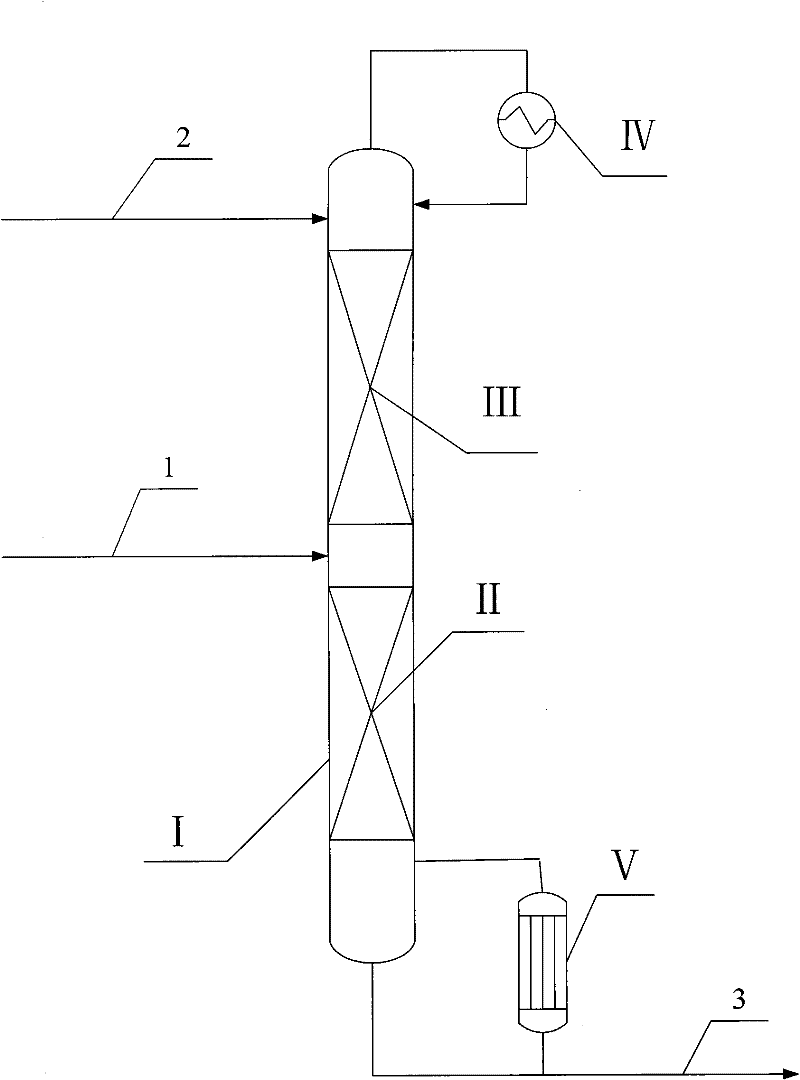
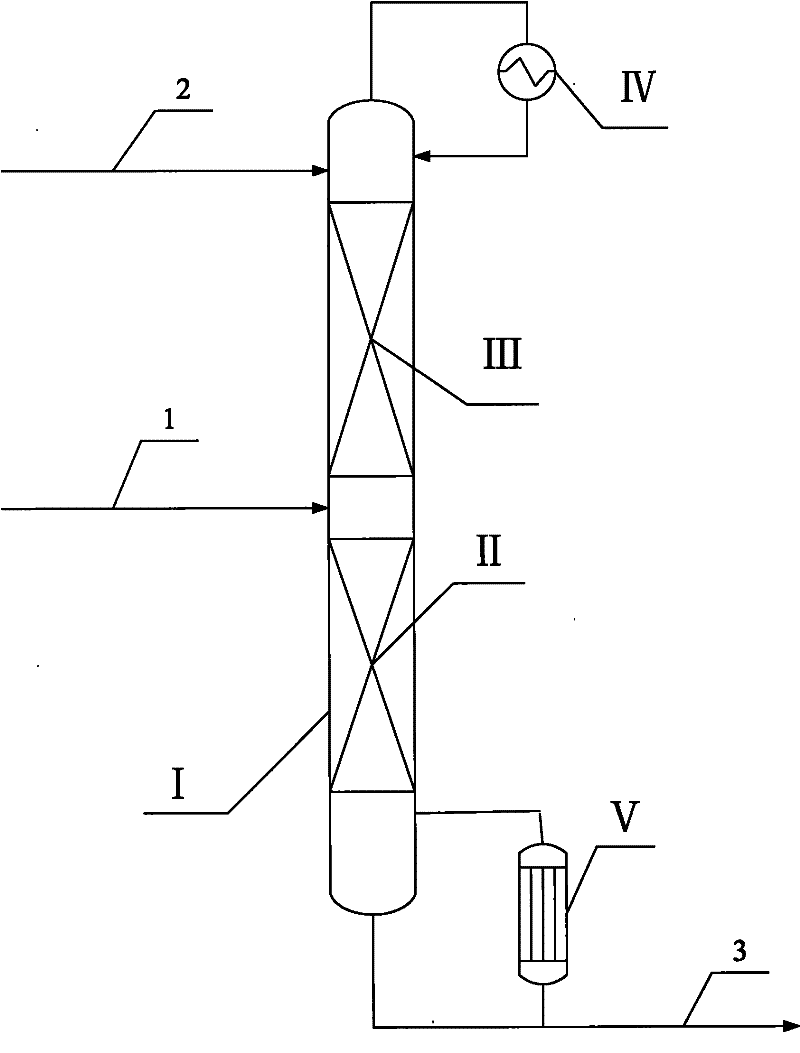
[0015] according to figure 1 As shown, the catalytic distillation tower operates under normal pressure, the number of theoretical plates in the stripping section is 15, the separation efficiency of the catalytic reaction section is equivalent to 18 theoretical plates, and the catalyst is SiO 2 / TiO 2 Titanium-silicon molecular sieve with a molar ratio of 80, 30% by weight of hydrogen peroxide and methanol mixture (methanol content 70% by weight) enters the first theoretical plate (counting from top to bottom, the same below) with a flow rate of 1 g / min, and allyl chloride is The flow rate of 2 g / min enters from the bottom of the catalytic reaction section, the temperature of the catalytic reaction section is 38-43°C, and the bottom of the tower is extracted at a flow rate of 3 g / min. It has been operated stably for 5000 hours. The catalytic distillation results are shown in Table 1.
Embodiment 2
[0017] according to figure 1 As shown, the catalytic distillation tower operates under normal pressure, the number of theoretical plates in the stripping section is 30, the separation efficiency of the catalytic reaction section is equivalent to 50 theoretical plates, and the catalyst is SiO 2 / TiO 2 Titanium-silicon molecular sieve with a molar ratio of 180, 50% by weight of hydrogen peroxide and acetone mixture (80% by weight of acetone content) enters the first theoretical plate with a flow rate of 1 g / min, and allyl chloride enters the first theoretical plate with a flow rate of 12 g / min from the bottom of the catalytic reaction section Enter, the temperature of the catalytic reaction section is 35-41°C, the bottom of the tower is extracted at a flow rate of 13 g / min, and it has been operated stably for 3000 hours. The catalytic distillation results are shown in Table 1.
Embodiment 3
[0019] according to figure 1 As shown, the catalytic distillation tower operates under normal pressure, the number of theoretical plates in the stripping section is 5, the separation efficiency of the catalytic reaction section is equivalent to 5 theoretical plates, and the catalyst is SiO 2 / TiO 2 Titanium-silicon molecular sieve with a mol ratio of 20, 2% by weight of hydrogen peroxide and ethyl acetate mixture (50% by weight of ethyl acetate content) enters the first theoretical plate with a flow of 1 g / min, and allyl chloride flows from the first theoretical plate with a flow of 5 g / min The bottom of the catalytic reaction section enters, the temperature of the catalytic reaction section is 42-46°C, and the bottom of the tower is withdrawn at a flow rate of 6 g / min. It has been operated stably for 1000 hours. The catalytic distillation results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com