2-benzimidazolyl-8-methanamide quinoline chromium complexes, its preparation method and application
A technology for complexes and polymerization reactions, applied in the field of 2-benzimidazolyl-8-carboxamide quinoline chromium complexes, can solve the problems that limit the development of ethylene oligomerization and polymerization industry, and achieve low price and high catalytic activity high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1, the preparation of complex C1
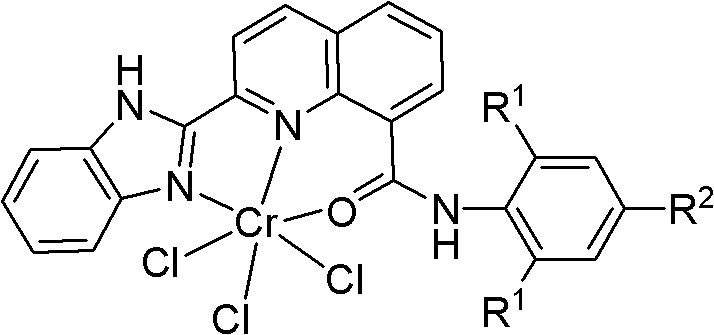
[0037] 1mmol tetrahydrofuran chromium chloride is added to the tetrahydrofuran solution containing 2-benzimidazolyl-N-(2,6-diisopropylphenyl)-8-carboxamide quinoline shown in 1.1mmol formula II, at 25 After stirring at ℃ for 6 hours, the reaction was completed, the solvent was sucked dry and washed several times with diethyl ether. The obtained precipitate was dried in vacuum to obtain a green solid powder, which is the complex represented by formula I provided by the present invention. Yield: 95.0%.
[0038]
[0039] (Formula I)
[0040] In the formula I, R 1 = i Pr, R 2 = H;
[0041] Theoretical value of elemental analysis: C 29 h 28 Cl 3 CrN 4 O: C, 57.39; H, 4.65; N, 9.23.; Found: C, 57.13; H, 4.55; N, 9.11. IR(KBr:cm -1 ): 3436m, 3210w, 3065m, 2966m, 2868w, 1623s, 1604s, 1570s, 1540m, 1442m, 1363w, 1339m, 1232w, 1146m, 1013w, 997m, 856w, 765m.
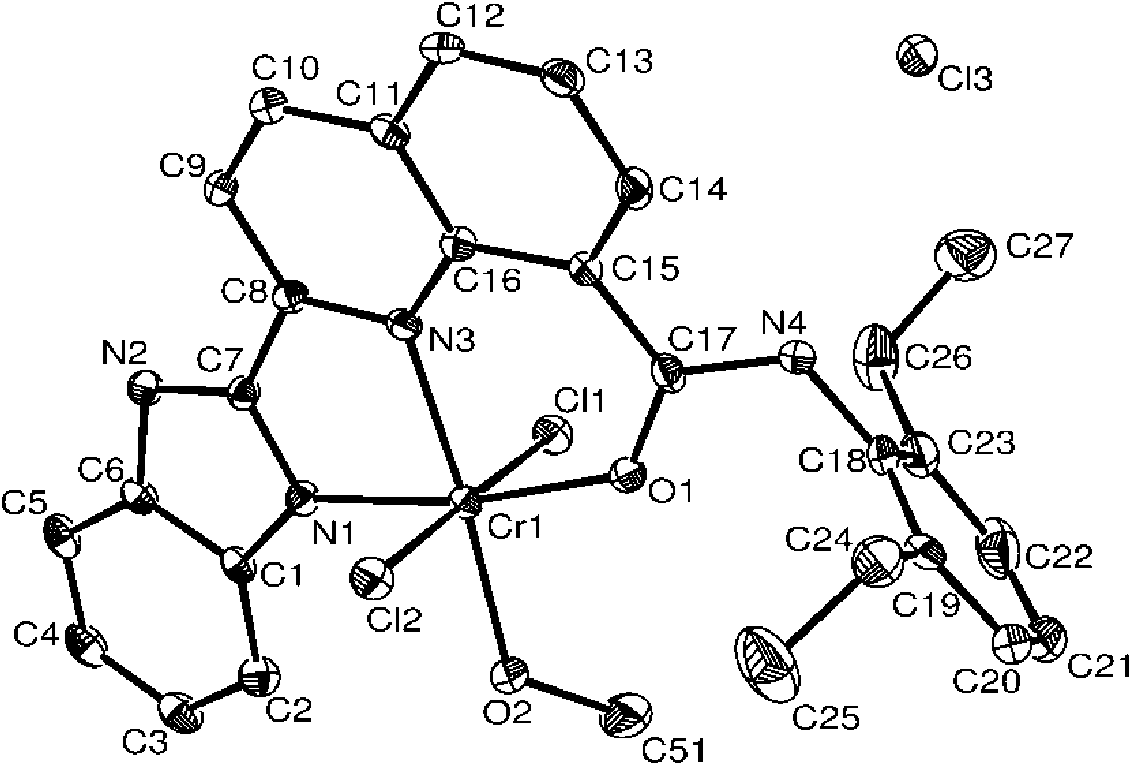
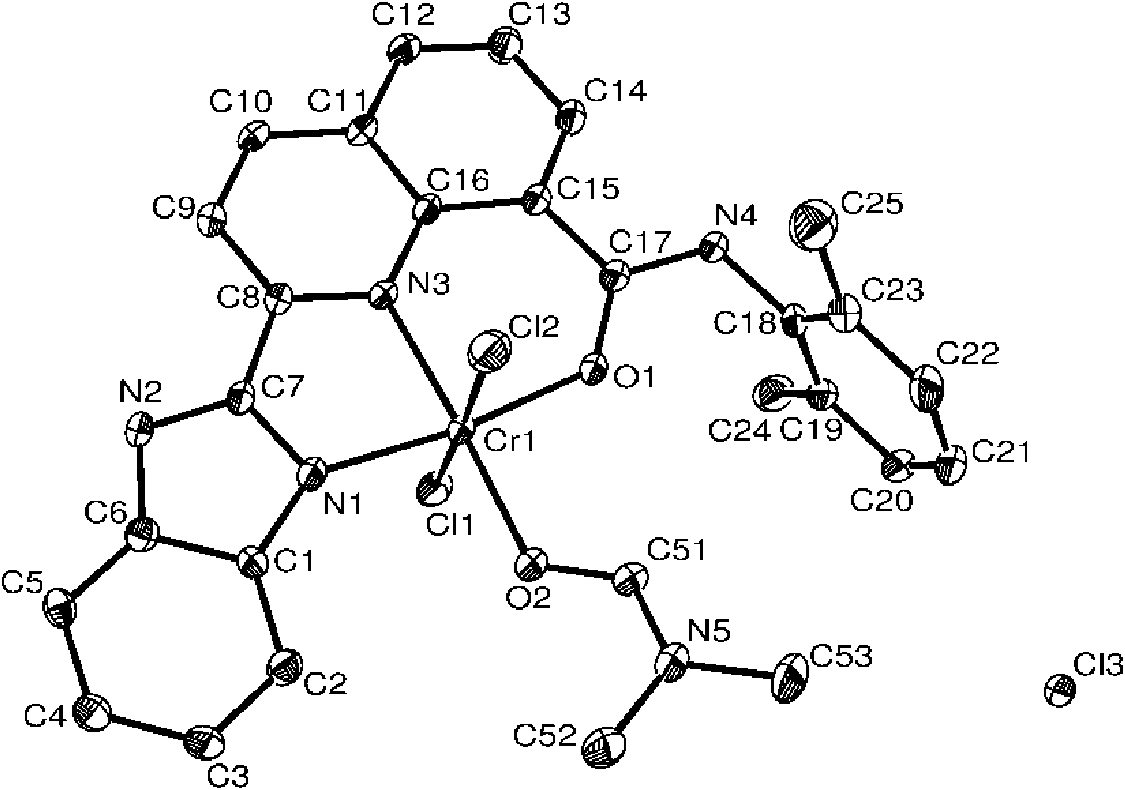
[0042] It can be known from the above that the compound has...
Embodiment 2
[0044] Embodiment 2, the preparation of complex C2
[0045] Add 1mmol tetrahydrofuran chromium chloride to tetrahydrofuran solution containing 1.1mmol formula II 2-benzimidazolyl-N-(2,6-diethylphenyl)-8-carboxamide quinoline, at 25°C After stirring for 6 hours, the reaction was completed, and the solvent was sucked dry and then washed several times with ether. The obtained precipitate was dried in vacuum to obtain a green solid powder, which is the complex represented by formula I provided by the present invention. Yield: 94.5%.
[0046]
[0047] (Formula I)
[0048] In the formula I, R 1 =Et,R 2 = H;
[0049] FT-IR (KBr, cm -1 ): 3416m, 3198m, 3065w, 2970m, 1623s, 1600s, 1565s, 1532m, 1501m, 1457w, 1340w, 1233w, 1141m, 1012m, 860m, 760m.Anal.Calcd for C 27 h 24 Cl 3 CrN 4 O: C, 56.02; H, 4.18; N, 9.68. Found: C, 55.98; H, 4.09; N, 9.65.
[0050] It can be known from the above that the compound has a correct structure and is a compound shown in formula I. figu...
Embodiment 3
[0052] Embodiment 3, the preparation of complex C3
[0053] Add 1mmol tetrahydrofuran chromium chloride to tetrahydrofuran solution containing 1.1mmol formula II 2-benzimidazolyl-N-(2,6-dimethylphenyl)-8-carboxamide quinoline, at 25°C After stirring for 6 hours, the reaction was completed, and the solvent was sucked dry and then washed several times with ether. The obtained precipitate was dried in vacuum to obtain a green solid powder, which is the complex represented by formula I provided by the present invention. Yield: 97.0%.
[0054]
[0055] (Formula I)
[0056] In the formula I, R 1 Me, R 2 = H;
[0057] FT-IR (KBr, cm -1 ): 3428m, 3185m, 3064w, 2925m, 1624s, 1601s, 1566s, 1538m, 1474w, 1440w, 1341w, 1303w, 1228w, 1150m, 1092m, 857m, 761m. 25 h 20 Cl 3 CrN 4 O: C, 54.51; H, 3.66; N, 10.17. Found: C, 54.50; H, 3.33; N, 10.10.
[0058] It can be known from the above that the compound has a correct structure and is a compound shown in formula I. figure 2 Pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com