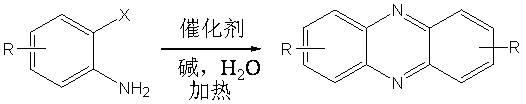
Method for preparing phenazine compound by catalyzing o-halogeno aniline in water phase
A technology of catalysts and in-situ catalysts, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, chemical/physical processes, etc., can solve problems such as dangerous operation, difficult control, and many by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
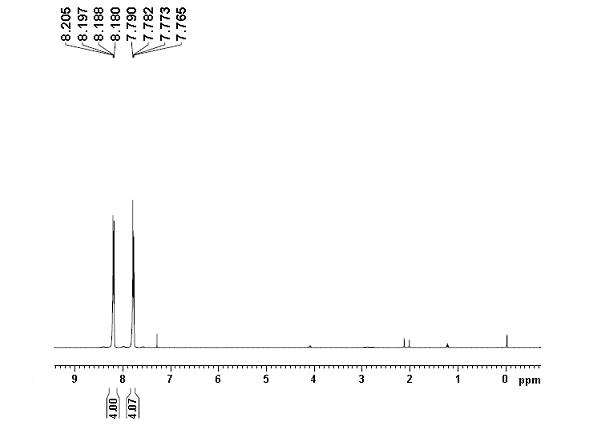
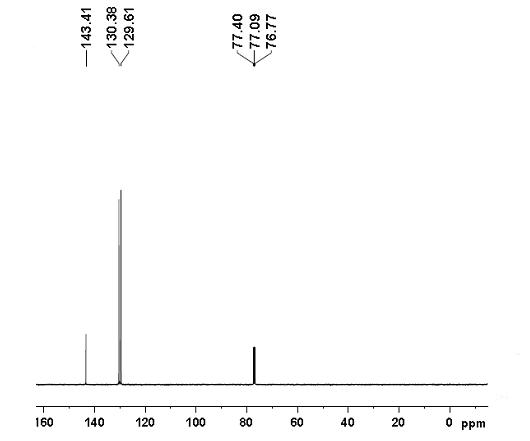
[0024] Example 1: Preparation of phenazine: 0.5 mmol (109.5 mg) of o-iodoaniline, 0.05 mmol (29.1 mg) of copper complex, 1 mmol (138 mg) of potassium carbonate, 0.1 mmol (64.4 mg), water 10 mL. at 120 o React in a C oil bath for 30 hours, cool to room temperature, extract the product with ethyl acetate, concentrate under reduced pressure, and purify the product by column chromatography to obtain a light yellow solid product with a yield of 78%. 1 H NMR (400 MHz, CDCl 3 ): δ = 7.77-7.79 (m, 4H), 8.18-8.21 (m, 4H) ppm (eg figure 1 ); 13 C NMR (100MHz, CDCl 3 ): δ = 129.6, 130.4, 143.4 ppm (eg figure 2 ). MS (EI, m / z ): 180[M + ].
Embodiment 2
[0025] Example 2: Preparation of 2,7-dimethylphenazine: The preparation method is the same as in Example 1, except that 0.5 mmol (116.5 mg) of 2-iodo-4-methylaniline is added, and the yield is 82%. 1 H NMR (400 MHz, CDCl 3 ): δ = 2.66 (s, 6H), 7.67 (d, 2H), 8.00 (s, 2H), 8.13 (d, 2H) ppm; 13 C NMR (100 MHz, CDCl 3 ): δ = 22.2, 127.6, 128.9, 133.4, 140.7, 142.2, 143.1 ppm. MS (EI, m / z ): 208[M + ].
Embodiment 3
[0026] Example 3: Preparation of 1,3,6,8-tetramethylphenazine: the preparation method is the same as in Example 1, except that 0.5 mmol (123.5 mg) of 2-iodo-4,6-dimethylaniline is added, and the yield 84%. 1 H NMR (400 MHz, CDCl 3 ): δ = 2.60 (s, 6H), 2.90 (s, 6H), 7.49 (s, 2H), 7.89 (s, 2H) ppm; 13 C NMR (100 MHz, CDCl 3 ): δ =17.6, 22.1, 126.0, 132.3, 136.7, 139.8, 141.5, 142.5 ppm. MS (EI, m / z): 236[M + ].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com