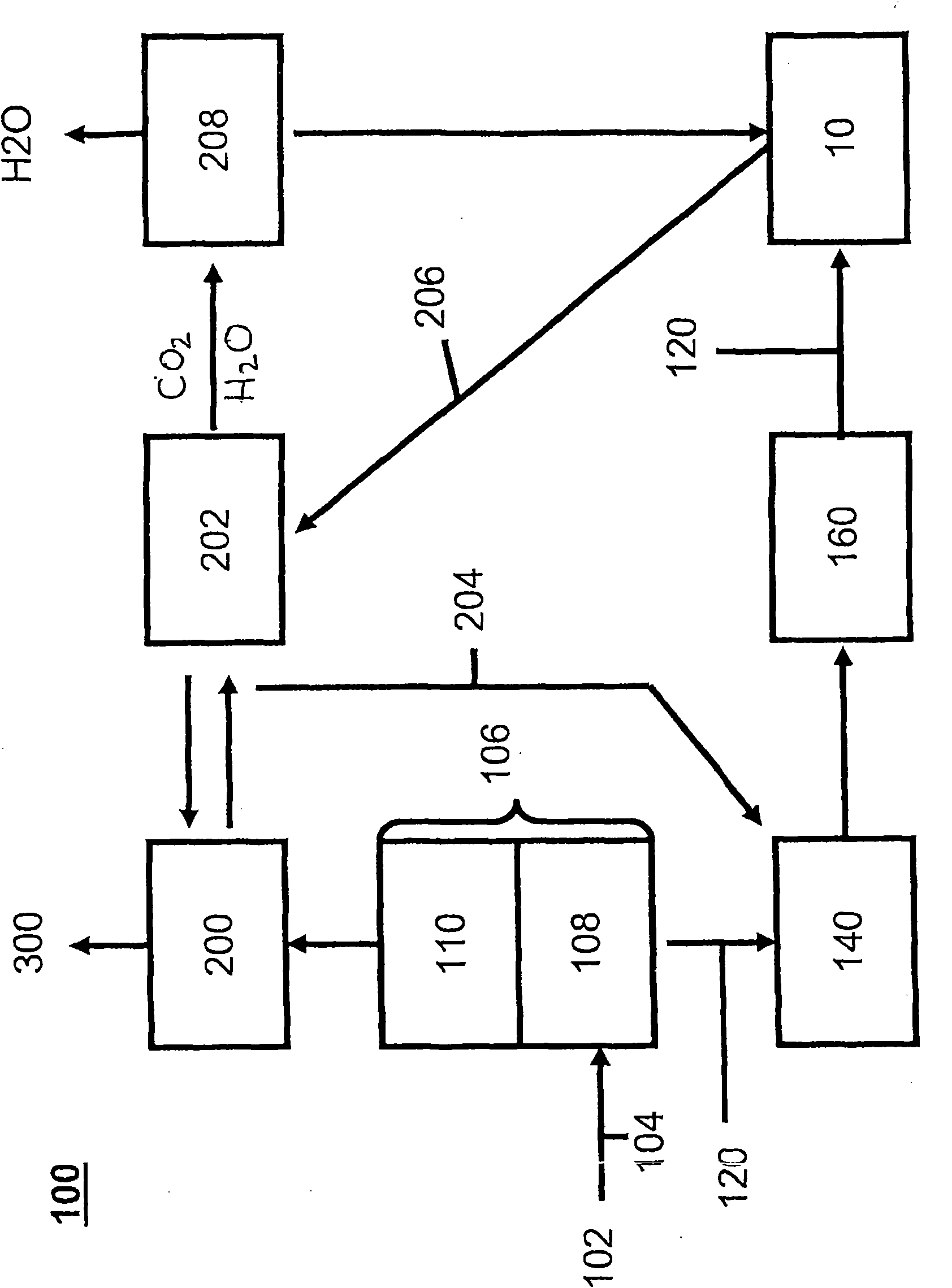
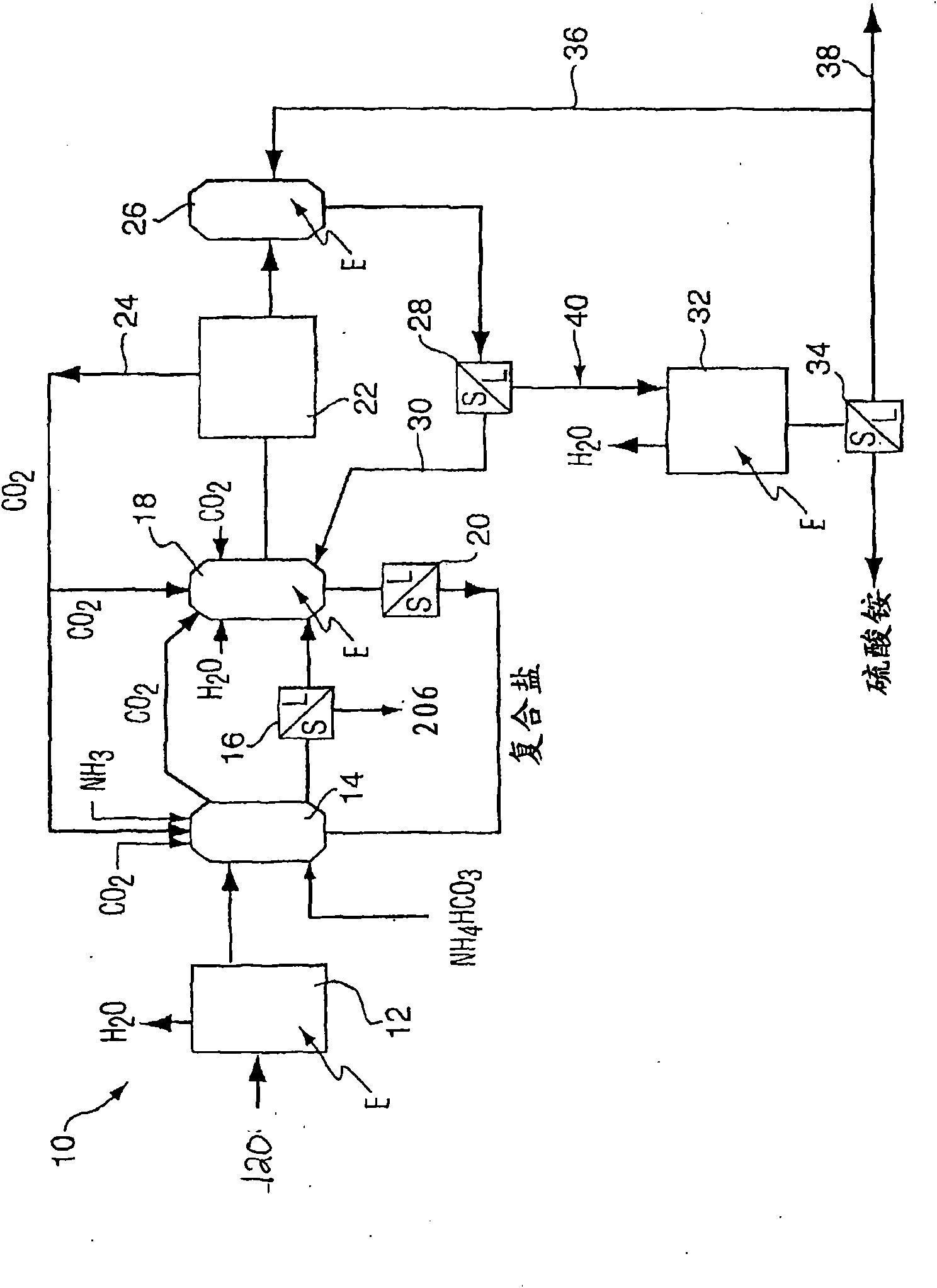
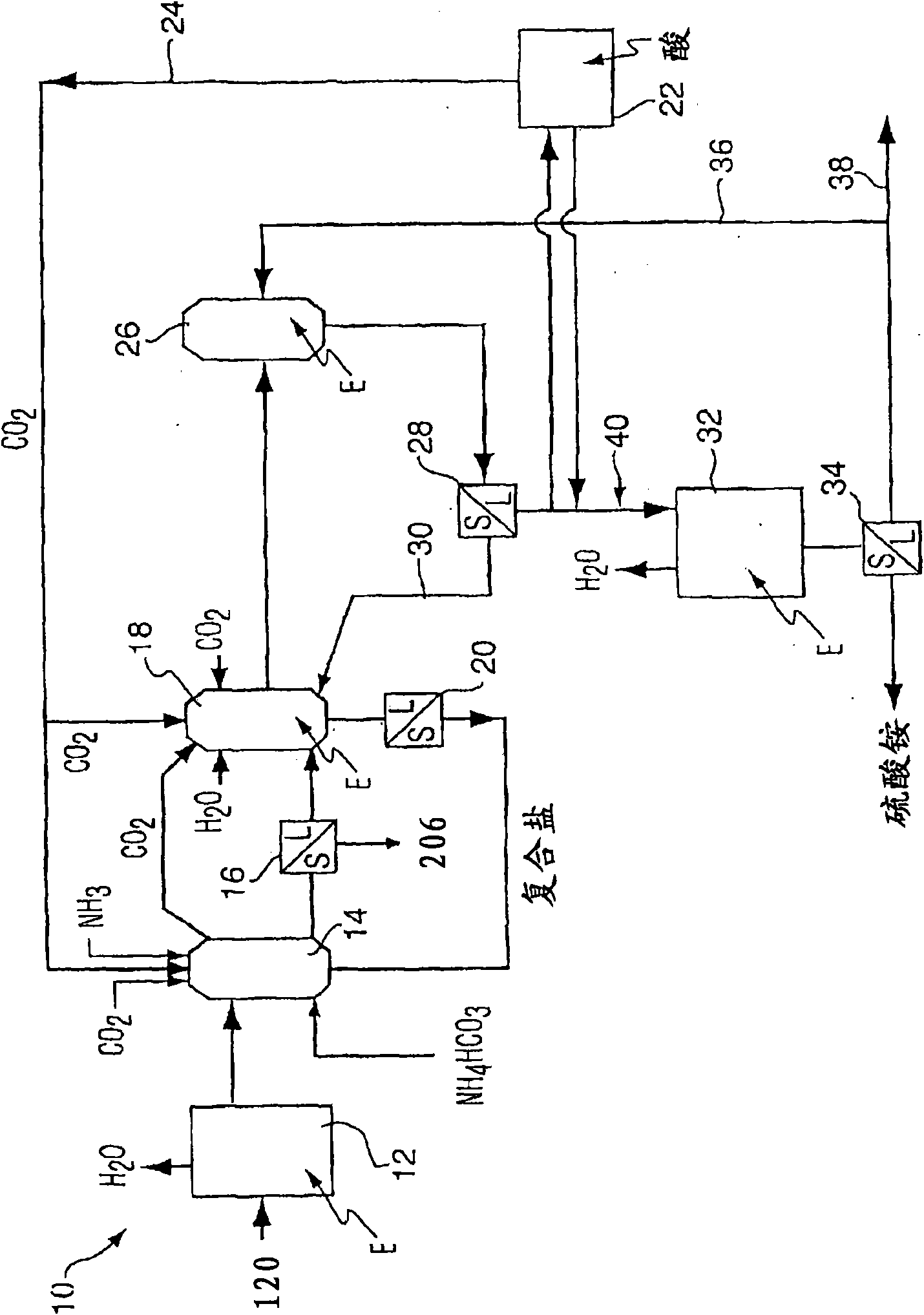
Method for purifying flue gas
A flue gas and flue technology, applied in chemical instruments and methods, separation methods, gas treatment, etc., can solve problems such as expensive investment and harmful by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Determination of the Optimal Ammonium to Sodium Ratio in the Sodium Bicarbonate Precipitation Step
[0045] The following examples illustrate how the complex phase equilibrium chemistry involved in the present invention can be used to determine the optimal ratio of ammonium to sodium in the sodium bicarbonate precipitation step. The understanding of chemistry demonstrated by this example is required for all unit operations within the process.
[0046] The following equilibrium reaction equation represents the process in the sodium bicarbonate precipitation step (using either solid ammonium bicarbonate or carbon dioxide and ammonia);
[0047]
[0048]
[0049] To understand the complexity of the phase equilibrium behavior described in this reaction, a schematic representation of the system is required. The balanced salt pair quaternary system described in this reaction can be represented on an isothermal "stereomodel". However, these dioramas are difficult to use...
example 1
[0081] Example 1: Molar ratio of ammonium to sodium = 1.0
[0082] If the feedstock has 1 mole of Na+ and the ammonium to sodium ratio is 1.0, the feedstock composition can be as follows:
[0083] Na + =1mol (23.0g)
[0084] NH 4 + =1mol (18.0g)
[0085] HCO 3 - =1mol (61.0g)
[0086] SO 4 2- = 0.5 mol (48.0 g).
[0087] Using Janecke and the law of levers, the mass of solid sodium bicarbonate produced was 53.7 g. It can be appreciated that the solid is 100% sodium bicarbonate. Therefore, Na + and HCO 3 - The conversion to sodium bicarbonate was 63.9%.
example 2
[0088] Example 2: Maximum single-pass sodium bicarbonate output (A / S molar ratio = 0.912)
[0089] as mentioned, Figure 6 Midpoint D represents the feedstock that will produce the maximum yield of sodium bicarbonate on a one-way basis. Point D has Janecke coordinates of (0.477, 0.523). If the feedstock has 1 mole of Na + , then it contains 0.5 moles of SO 4 2- . NH 4 + and HCO 3 - The number of moles of is equal and can be obtained from:
[0090] 0.523 = Na + moles / (Na + moles+NH 4 + moles)
[0091] or
[0092] 0.477 = HCO 3 - moles / (HCO 3 - Number of moles+(2×SO 4 2- moles)
[0093] These give 0.912 moles of NH 4 + and HCO 3 - and a molar ratio of ammonium to sodium of 0.912. Therefore, we will have a feedstock with the following composition:
[0094] Na + =1mol (23.0g)
[0095] NH 4 + =0.912mol (16.4g)
[0096] HCO 3 - =0.912mol (55.6g)
[0097] SO 4 2- =0.5mol (48.0g)
[0098] Sum = 143.0g
[0099] As before, using Janecke and the la...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com