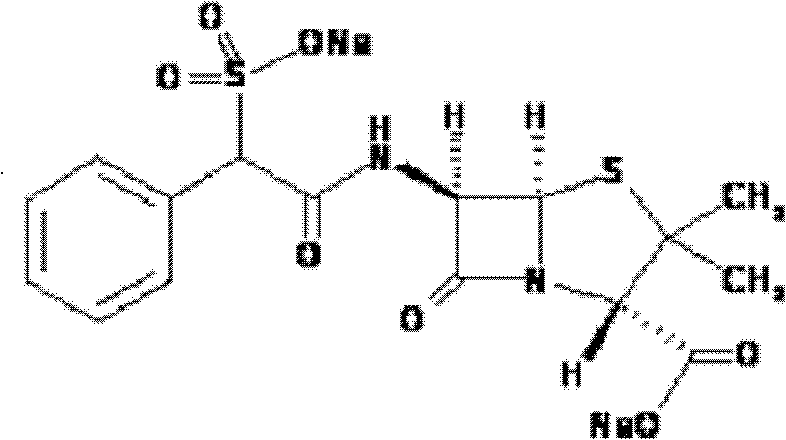
Method for purifying D (-)-sulbenicillin sodium
A technology of sulfobenicillin sodium and a purification method, which is applied in the field of β-cyclodextrin purification of D-sulfobenicillin sodium, can solve the problems of easy racemization of levoform, complicated process, inability to use, etc., and achieves the effect of improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] (a) mixing and dissolving the crude sulfobenicillin sodium with water in a weight ratio of 2:1;
[0054] (b) The solution temperature was maintained at 30°C through a porous ceramic tube with a pore size of 60 μm loaded with β-cyclodextrin membrane, and the solution flow rate was 6 dl / sec;
[0055] (c) add dehydrated alcohol of 1 / 2 of Sulfonbenicillin Sodium crude product weight and the isopropanol of Sulfonbenicillin Sodium crude product weight, stir and grow crystal in reaction tank until there is a large amount of crystals to separate out, open frozen brine and cool down 8h;
[0056] (d) centrifugal separation to obtain D(-)-sulfobenicillin sodium.
[0057] The yield of sulfenbenicillin sodium was 60%, and the content of D(-)-sulfenbenicillin sodium was 80%. The optical rotation of the product is determined to be [α]D: +160°~+180°.
Embodiment 2
[0059] (a) mixing and compatibilizing sulfobenicillin sodium crude product with water at a weight ratio of 9:1;
[0060] (b) A ceramic tube with a pore size of 2 μm passed through a loaded L-lysine-β-cyclodextrin membrane at room temperature, and the solution flow rate was 2 dl / sec;
[0061] (c) add dehydrated alcohol of 1 / 2 of Sulfonbenicillin Sodium crude product weight and the isopropanol of Sulfonbenicillin Sodium crude product weight, stir and grow crystal in reaction tank until there is a large amount of crystals to separate out, open frozen brine and cool down 9h;
[0062] (d) centrifugal separation to obtain D(-)-sulfobenicillin sodium.
[0063] The yield of Sulfonicillin Sodium was 65%, and the content of D(-)-Sulfonbenicillin Sodium was 99%. The optical rotation of the product is determined to be [α]D: +175°~+180°.
[0064] NMR test results of the obtained product: 1H-NRM (400MHz, D2O)δ(ppm): 1.4025(t, 6H, -CH3), 4.1153(d, H, -CH-COONa), 4.9275(s, H, Ar- CH), 5.46...
Embodiment 3
[0066] (a) mixing and compatibilizing sulfobenicillin sodium crude product with water at a weight ratio of 7:1;
[0067] (b) Passing through a carboxymethyl-β-cyclodextrin membrane loaded with a carboxymethyl-β-cyclodextrin membrane while keeping the solution temperature at 27°C, a ceramic tube with a pore size of 100 μm and a solution flow rate of 10 dl / sec;
[0068] (c) add the dehydrated alcohol of 1 / 2 of the Sulfonicillin Sodium crude product weight and the isopropanol of Sulfonbenicillin Sodium crude product weight, stir and grow crystals in the reaction tank until a large amount of crystals separate out, open the frozen brine and cool down 10h;
[0069] (d) centrifugal separation to obtain D(-)-sulfobenicillin sodium.
[0070] The yield of sulfobenicillin sodium was 50%, and the content of D(-)-sulfobenicillin sodium was 93%. The optical rotation of the product is determined to be [α]D: +170°~+180°.
[0071] It can be seen from the above that the optical rotation range...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com