Cis-nitenpyram compound including 1,3-dicarbonyl, preparation method and use
A technology of nitenpyram and dicarbonyl, which is applied in the field of cis-nitenpyram analogs and their preparation, can solve the problems of light instability and poor hydrophobicity, limitations in promotion and application, serious pest resistance, etc. Significant practicability, good insecticidal effect, and short response time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
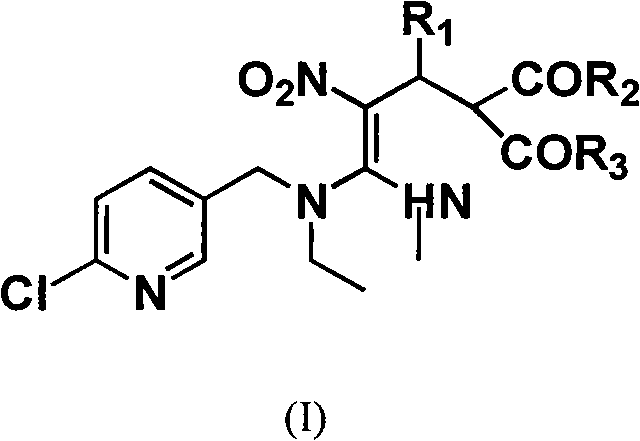
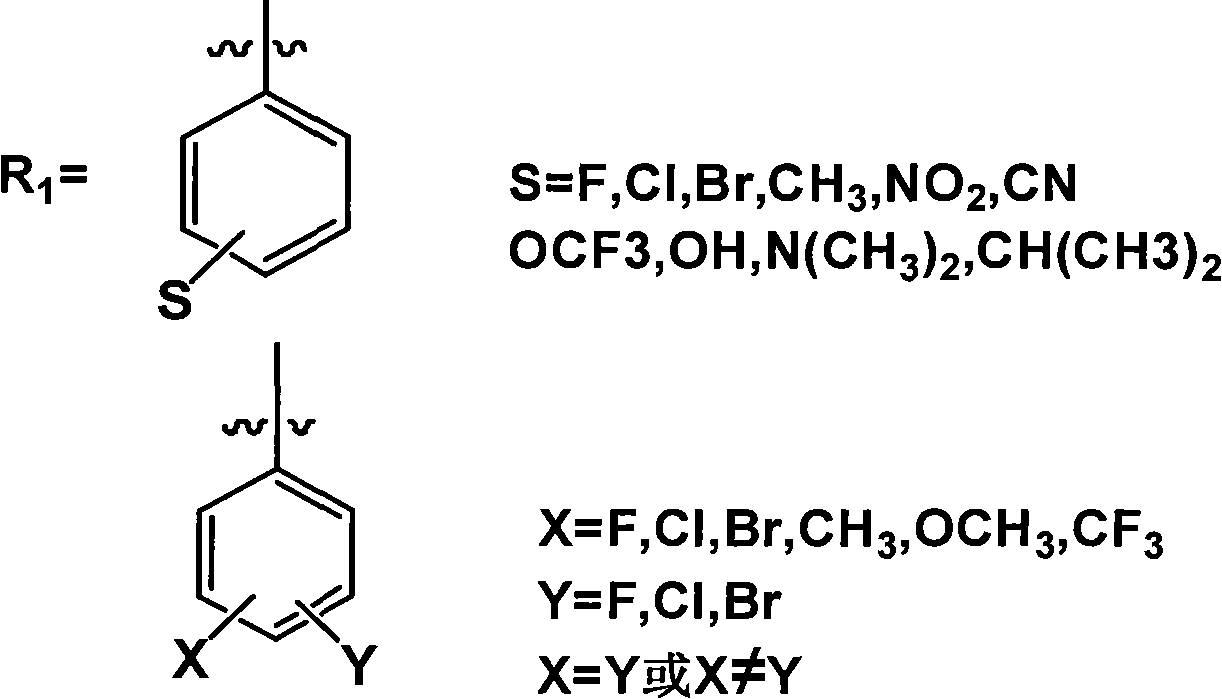
[0046] Preparation of cis-{3-[N-(6-chloro-3-pyridylmethyl)-N-ethyl]amino-3-N-methylamino-2-nitro-1-(2,4-dichloro phenyl)-allyl}-2,4-pentanedione.
[0047] Add 30mL of absolute ethanol to a 250mL three-necked flask, then add 15mmol of acetylacetone, 15mmol of 2,4-dichlorobenzaldehyde, 10mmol of nitenpyram, and then add 6 drops of piperidine as a catalyst. Heat the round-bottomed three-neck flask containing the above solution in an oil bath, control the temperature at about 78°C, reflux for 6 hours, remove absolute ethanol under reduced pressure, add 10mL of water to dissolve and extract three times with 60mL of ethyl acetate. The combined extracts were dried overnight with anhydrous magnesium sulfate, and the solvent was distilled off to obtain a yellow oil. Column chromatography with ethyl acetate:petroleum ether=1:2 gave a pale yellow solid with a yield of 82%.
[0048] Elemental analysis: found value C% 55.94 H% 5.28 N% 11.32
[0049] Calculated C% 55.99 H% 5.31...
Embodiment 2
[0053] Preparation of cis-{3-[N-(6-chloro-3-pyridylmethyl)-N-ethyl]amino-3-N-methylamino-2-nitro-1-(3,4-dichloro phenyl)-allyl}-2,4-pentanedione.
[0054] Add 30 mL of absolute ethanol to a 250 mL three-necked flask, then add 15 mmol of malononitrile, 15 mmol of 3,4 dichlorobenzaldehyde, 10 mmol of nitenpyram, and then add 6 drops of piperidine as a catalyst. Heat the round-bottomed three-neck flask containing the above solution in an oil bath, control the temperature at about 78°C, reflux for 6 hours, remove absolute ethanol under reduced pressure, add 10mL of water to dissolve and extract three times with 60mL of ethyl acetate. . The combined extracts were dried overnight with anhydrous magnesium sulfate, and the solvent was distilled off to obtain a yellow oil. Column chromatography with ethyl acetate:petroleum ether=1:1 gave a light yellow solid with a yield of 76.0%.
[0055] Elemental analysis: found value C% 52.28 H% 4.74 N% 10.62
[0056] Calculated C% 52...
Embodiment 3
[0060] Preparation of cis-{3-[N-(6-chloro-3-pyridylmethyl)-N-ethyl]amino-3-N-methylamino-2-nitro-1-phenyl-allyl} -2,4-pentanedione.
[0061] Add 30 mL of absolute ethanol to a 250 mL three-necked flask, then add 15 mmol of acetylacetone, 15 mmol of benzaldehyde, and 10 mmol of nitenpyram in sequence, and then add 6 drops of piperidine as a catalyst. Heat the round-bottomed three-neck flask containing the above solution in an oil bath, control the temperature at about 78°C, reflux for 6 hours, remove absolute ethanol under reduced pressure, add 10mL of water to dissolve and extract three times with 60mL of ethyl acetate. The combined extracts were dried overnight with anhydrous magnesium sulfate, and the solvent was distilled off to obtain a yellow oil. Column chromatography with ethyl acetate:petroleum ether=1:1 gave a light yellow solid with a yield of 79.1%.
[0062] Elemental analysis: found value C% 50.57 H% 3.67 N% 17.08
[0063] Calculated C% 51.08 H% 3.88 N...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com