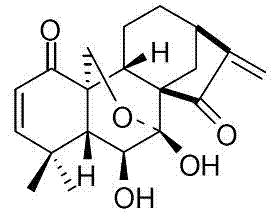
Application of eriocalyxin to preparation of medicaments for treating autoimmune diseases
An autoimmune, calyxine technology, applied in allergic diseases, drug combinations, nervous system diseases, etc., can solve the problems of unreported application of calyxe
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
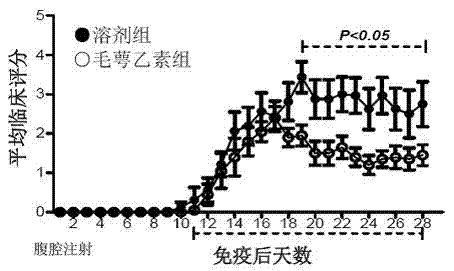
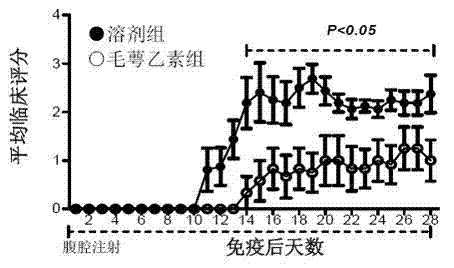
[0091] Example 1 Calyxin alleviates the condition of EAE in mice
[0092] (A) Treatment options:
[0093] Establish the EAE mouse model. After the onset of the disease (the 11th day after the establishment of the model), the mice were randomly divided into groups, and intraperitoneally injected with solvent (200 μl / mouse, solvent control group, n=8) or B. (administration group, n=10), the dose was 10 mg echinoceline / kg body weight, for 18 consecutive days, the state of the mice was observed every day, and clinical scores were made according to the degree of disease. The mice in the solvent control group all broke out the typical EAE condition, and the average highest clinical score reached 3.81±0.34, while the average highest clinical score in the treatment group was 2.80±0.29.
[0094] (B) Prevention program:
[0095] After the mice were randomly grouped, they were given solvent (200 μl / mouse, solvent control group, n=8) or echinacea (administration group, n=6) every day ...
Embodiment 2
[0097] Example 2 Pathological observation of the spinal cord of EAE mice after treatment with calyxin
[0098] (A) H&E staining
[0099] H&E tissue section staining was performed on the spinal cord lesions of the normal mice, EAE-onset mice in the solvent group and the drug-administered group. There was no inflammatory infiltration in normal mice, and there were a large number of inflammatory infiltration cells in the perivascular tissue of the spinal cord of the mice in the solvent group, while the inflammatory infiltration in the corresponding parts of the mice in the echinoceline B treatment group was significantly reduced.
[0100] (B) Luxol Fast Blue staining
[0101] For normal mice, EAE-onset mice in vehicle group and administration group, the spinal cord lesions were stained with Luxol Fast Blue myelin tissue sections. Normal mice had no demyelinating lesions, and the white matter part of the spinal cord of the mice in the solvent group was largely lost, and the va...
Embodiment 3
[0103] Example 3 Calyxin inhibits the proliferation and differentiation of autoantigen-specific T cells in EAE mice
[0104] Under the specific inflammatory microenvironment and corresponding antigen stimulation, naive CD4+ T cells are activated and differentiated into various helper T cells (Th) to mediate effector effects. Th1 and Th17 cells play a pathogenic role in the pathogenesis of EAE, and regulatory T cells (Treg) have an inhibitory effect on the pathogenesis of EAE. In order to reveal the mechanism of EriB in protecting EAE mice, we first detected the proliferative ability of the EriB-treated group and the solvent control group in mice stimulated against the self-antigen MOG at the peak of EAE. The results showed that normal mice were resistant to MOG No response; EriB splenocytes of the mice in the solvent group had strong proliferative responses to MOG stimulation; T cells in the peripheral lymphoid organs (spleen) of the mice treated with eriocalyxin were signif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com