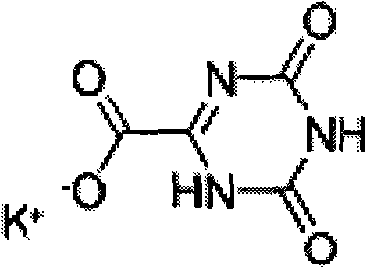
Preparation method suitable for industrially producing oteracil potassium
A technology of oteracil potassium and potassium iodide, applied in the field of medicinal chemistry, can solve the problems of undisclosed final product purity and the like, and achieve the effects of reducing the use of organic solvents, saving costs, and having a simple refining method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Dissolve 560g of potassium hydroxide in 2000ml of deionized water, cool to 0-5°C, add 8g of potassium iodide, cool to 0-5°C, add 158g of allantoin, slowly add 320g of bromine, and control the temperature at 1-5°C Reaction, after the dropwise addition, raise the temperature to 20°C and react for 30-40 hours until the reaction is complete, adjust the pH to 6±0.2 with glacial acetic acid while stirring, cool down to 0-5°C, stir and crystallize for one hour, filter with suction, add deionization to the solid 1000ml of water was stirred into a suspension, filtered with suction, and the filter cake was repeatedly washed with deionized water once, and dried to obtain 145g of crude product with a yield of 74%. The purity by HPLC was 98.5%, and there was one impurity with a content greater than 0.5%.
[0018] Add 2 L of deionized water to the crude product of oteracil potassium, slowly add 50% potassium hydroxide aqueous solution and stir until clarification, filter to remove ins...
Embodiment 2
[0020] Dissolve 510g of potassium hydroxide in 2000ml of deionized water, cool to 0-10°C, add 10g of potassium iodide, cool to 0-5°C, add 158g of allantoin, slowly add 400g of bromine, and control the temperature at 1-5°C Reaction, after the dropwise addition, raise the temperature to 20°C and react for 30-40 hours until the reaction is complete, adjust the pH to 6±0.2 with 15% hydrochloric acid under stirring, cool down to 0-5°C, stir and crystallize for one hour, filter with suction, and remove the solids 1000ml of deionized water was stirred into a suspension, suction filtered, and the filter cake was repeatedly washed with deionized water once, and dried to obtain 140g of crude product, with a yield of 71%, a purity of 98.7% by HPLC, and one impurity with a content greater than 0.5%;
[0021] Add 2 L of deionized water to the crude product of oteracil potassium, slowly add 50% potassium hydroxide aqueous solution and stir until clarification, filter to remove insoluble matt...
Embodiment 3
[0023] Dissolve 610g of potassium hydroxide in 2000ml of deionized water, cool to 5-10°C, add 13g of potassium iodide, cool to 0-5°C, add 158g of allantoin, slowly add 450g of bromine, and control the temperature at 1-5°C Reaction, after the dropwise addition, raise the temperature to 20°C and react for 30-40 hours until the reaction is complete, adjust the pH to 6±0.2 with 15% sulfuric acid under stirring, cool down to 0-5°C, stir and crystallize for 2 hours, filter with suction, and remove the solids 1000ml of deionized water was stirred into a suspension, suction filtered, and the filter cake was repeatedly washed with deionized water once, and dried to obtain 142g of crude product, with a yield of 73%;
[0024] Add 2 L of deionized water to the crude product of oteracil potassium, slowly add 50% potassium hydroxide aqueous solution and stir until clarification, filter to remove insoluble matter, add 15% sulfuric acid dropwise to the filtrate under stirring to adjust the pH ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com