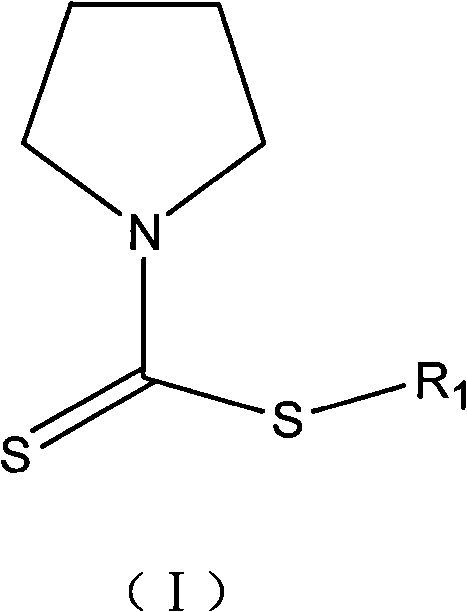
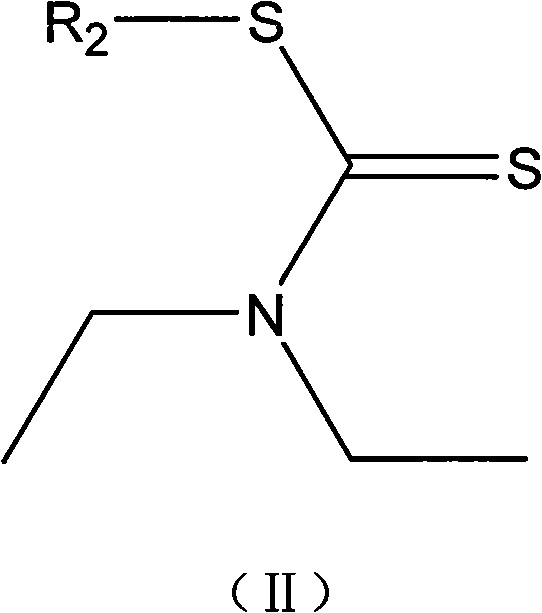
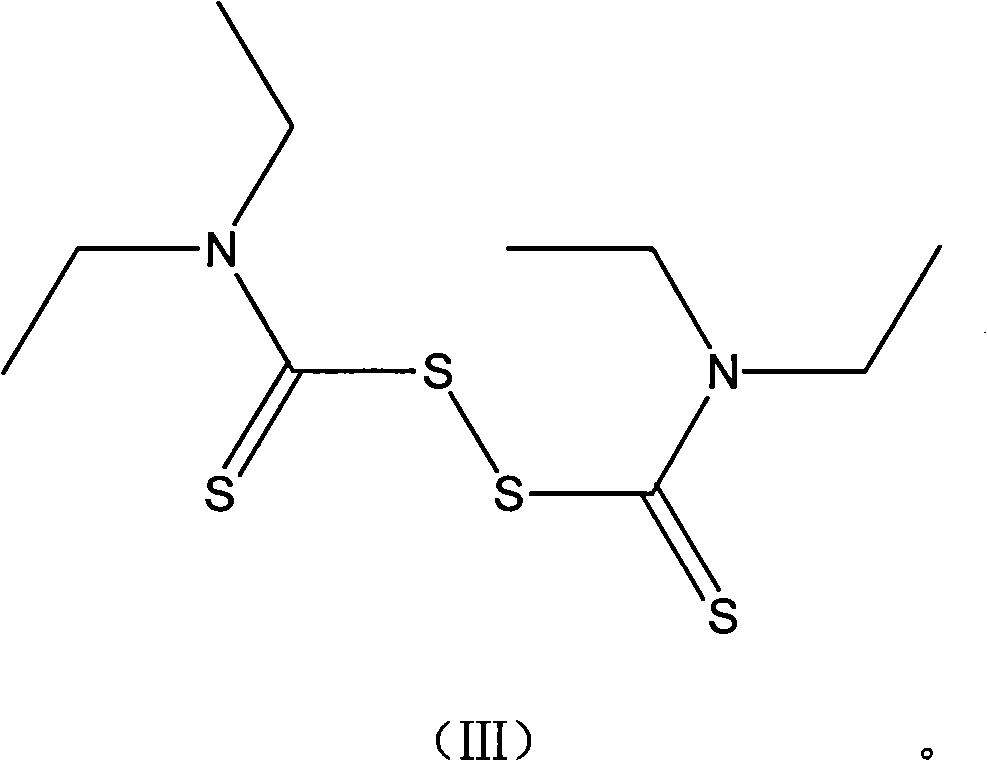
Mycobacterium tuberculosis resisting compounds, and applications thereof
A technology of mycobacteria and compounds, applied in the field of medicine, can solve the problems of difficult treatment, unsatisfactory cure rate, increased toxicity of therapeutic drugs, etc., and achieve the effect of enhancing antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1 Mycobacterium tuberculosis is to the in vitro drug susceptibility result determination of PDTC and DETC
[0044] (1) Experimental method:
[0045] (1) Inoculation method of Mycobacterium tuberculosis: Get Mycobacterium tuberculosis H37Rv (ATCC 25618) and H37Ra (ATCC25177) bacterial strains, inoculate in containing 10% ADC, 0.5% glycerol and 0.05% Tween 80 (Sigma-Aldrich, St.Louis , MO) in Middlebrook 7H9 medium, cultured at 37°C.
[0046] (2) Determination of the minimum inhibitory concentration (MIC): Inoculate the H37Rv cultured for 2 weeks into 5 ml Middlebrook 7H9 medium containing 10% ADC and 0.5% glycerol (~6.8pH) at a ratio of 1:100, 37 Cultivate for two weeks. At the same time, add PDTC or DETC diluted by 2 times to form a series of concentration gradients. The minimum inhibitory concentration (MIC) was determined by visually observing the size of the cell particles between the cultures with different dilutions of the drug and the control group w...
Embodiment 2
[0051] Embodiment 2: Determination of serum inhibitory titers after oral administration of corresponding compounds in mice
[0052] (1) Experimental method:
[0053] Mice were given corresponding drugs by intragastric administration, respectively 0.2ml sterile water (control), water containing PDTC (100mg / kg) and DETC (100mg / kg) and water containing INH (25mg / kg). After 1 hour, the blood of mice fed with INH was collected; after 2 hours, the blood of mice fed with PDTC and DETC was collected. Since there were 5-6 mice in each drug group, the sera obtained from mice in each group were pooled together. Serial dilutions of serum (1:2 to 1:64) were then prepared in 7H9 medium containing glycerol and ADC. The two-week-old H37Rv was inoculated into serum at a ratio of 1:100. At 1, 2, and 3 weeks after inoculation, the inhibition of bacterial growth in the test tube was observed with the naked eye. Inhibitory titers were determined by colony forming unit counts in Middlebrook 7H1...
Embodiment 3
[0058] Determination of antibacterial activity to growth phase and stationary phase Mycobacterium tuberculosis under embodiment 3 acidic and neutral conditions
[0059]Apply growth phase bacteria cultured for 21 days and old stationary phase bacteria cultured for 67 days, inoculate in liquid culture containing PDTC (20 μM, equivalent to 3.3 μg / ml) or DETC at pH 5.5 (acidic) or 7.0 (neutral) cultured in medium for 3 days. The results showed that after short-term drug exposure, CFU counts of surviving bacteria showed that PDTC had an effect on both growth and stationary phase bacteria under neutral and acidic culture conditions. PDTC and DETC can kill growth phase bacteria under acidic and neutral culture conditions and stationary phase bacteria under neutral culture conditions. In addition, DETC had stronger antibacterial activity than PDTC for stationary bacteria under acidic culture conditions. At neutral pH, PDTC was more active against growth-phase bacteria. In addition,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com