Drug used for preventing Mycobacterium tuberculosis and Mycobacterium tuberculosis infection, and applications thereof
A Mycobacterium tuberculosis and anti-tuberculosis technology, applied in the field of biomedicine, can solve the problems of undiscovered polypeptide drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1. Action molecule is selected colistin Ia, prepares recombinant polypeptide
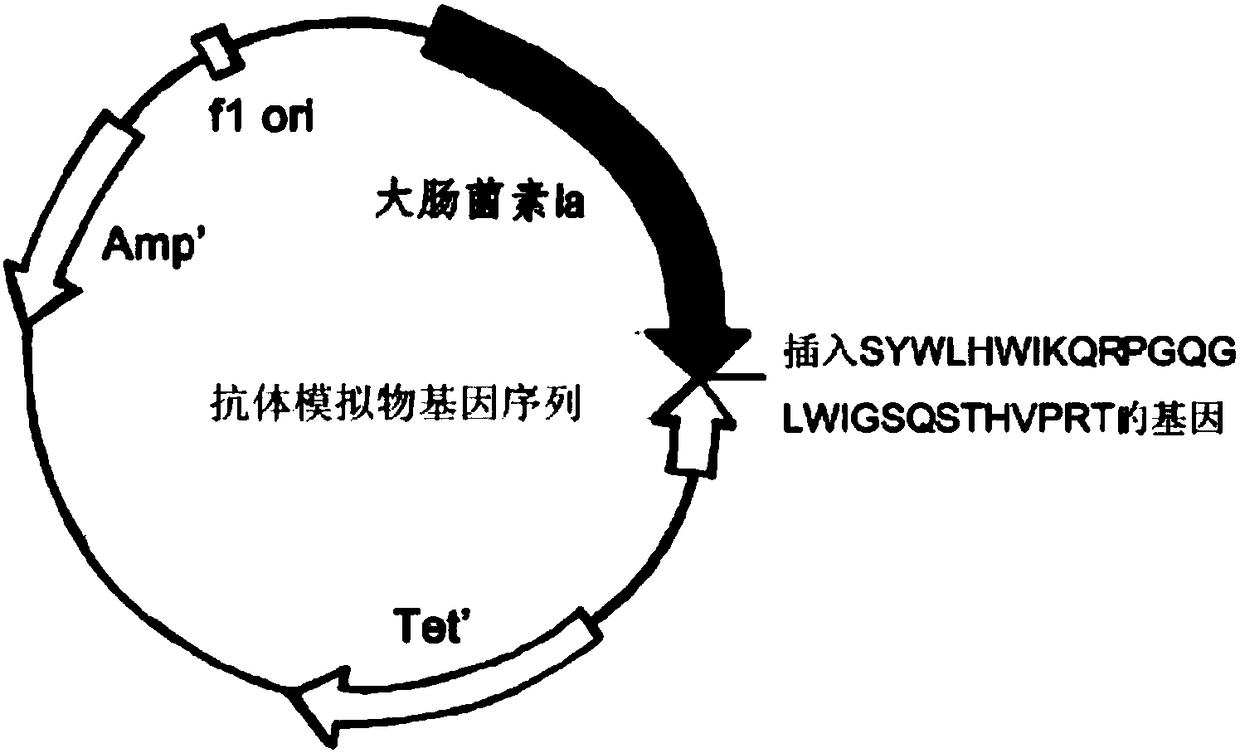
[0055] The original plasmid is pSELECT loaded with colicin and immunity protein genes TM -1 plasmid (8.3kb). Double-stranded oligonucleotide point mutation technology (QuickChange TM Kit, Strategene Company) will encode the gene fragment of the antibody mimetic: 5-tcttattggctgcattggat taaacagaga cctggtcagg gactgtggatcggatctcagtccacgcatg tgccgagaacc-3 (Seq ID No.1) or 5-tcttattggc tgcattggattaaacagaga cctggtcagg gactgtggat cggaaccaga ccggt3gcata cg) into (Seqaccaga ccggt3gcata cg) On the 626 position of the colicin polypeptide gene, obtain the mutant plasmid pBHC-PorA1 and pBHC-PorA2 (such as figure 1 - figure 2 shown). The mutant plasmid was transfected into E.coli BL-21 engineering bacteria to prepare new antibiotics.
[0056] The mutation program was carried out according to the Strategene QuickChange SiteDirected Mutagenesis Kit (catalog#200518) kit manual, namely:
[00...
experiment example 1
[0102] Experimental Example 1 Contrastive Experiment on the Minimum Inhibitory Concentration of Lethal Drug-resistant Strains
[0103] Experimental strain:
[0104] Susceptible strains: Mtb Erdman is susceptible to current anti-tuberculosis drugs, SUNY Upstate Medical University
[0105] Drug-resistant strains: PUMC-94789 is a strain of the Beijing genotype, resistant to isoniazid and rifampicin, with the classic isoniazid-resistant katG315 gene mutation and rpoB531,526 gene mutation resistant to rifampicin, PUMC-94789 The virulence is stronger, and its LD50 mouse survival period is 7 days (the tuberculosis standard strain H37Rv survival period is 14 days). Peking Union Medical College Peking Union Medical College.
[0106] 506 strains of multidrug-resistant tuberculosis (MDR-TB) were provided by the National Laboratory of Tuberculosis Reference, China CDC.
[0107] The above-mentioned strains are well-known strains, and the applicant's laboratory also preserves them. An ap...
experiment example 2
[0122] Experimental Example 2 Counting of Bacterial Colonies in the Lungs of Mouse Pulmonary Tuberculosis Infection Model
[0123] Female BLAB / c mice were inoculated nasally with 6.8x 10 2 CFU Mtb Erdman tuberculosis;
[0124] Each group of 6 rats was divided into five groups: early control group, late control group, isoniazid group, low-dose pheromone group, high-dose pheromycin group;
[0125] After 21 days of infection, mice in each group were treated as follows:
[0126] Early control group and late control group were intraperitoneally injected with normal saline;
[0127] The isoniazid group was given oral isoniazid 183μmol / kg / d (25mg / kg / d);
[0128] Low-dose pheromycin group and high-dose pheromycin group were injected intraperitoneally with 0.286 or 0.572 μmol / kg / d (20 or 40 mg / kg / d) pheromone, respectively.
[0129] Four weeks later, the right lung was removed, ground, homogenized and cultured to count tuberculosis colonies. The statistical results were as follows:...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com