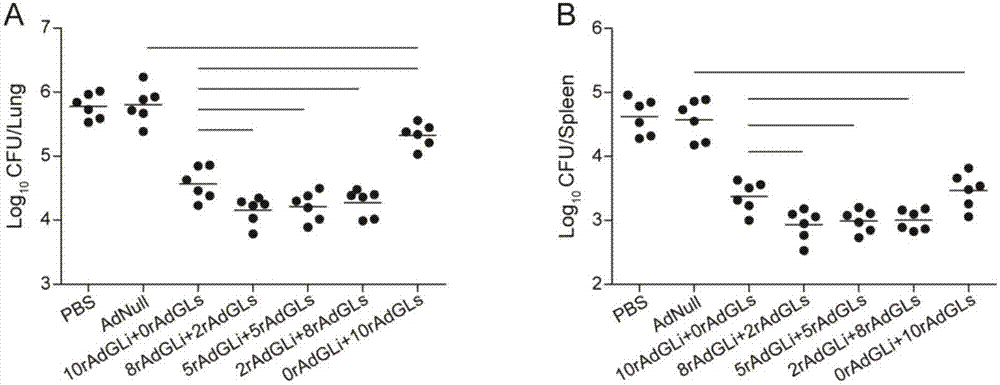
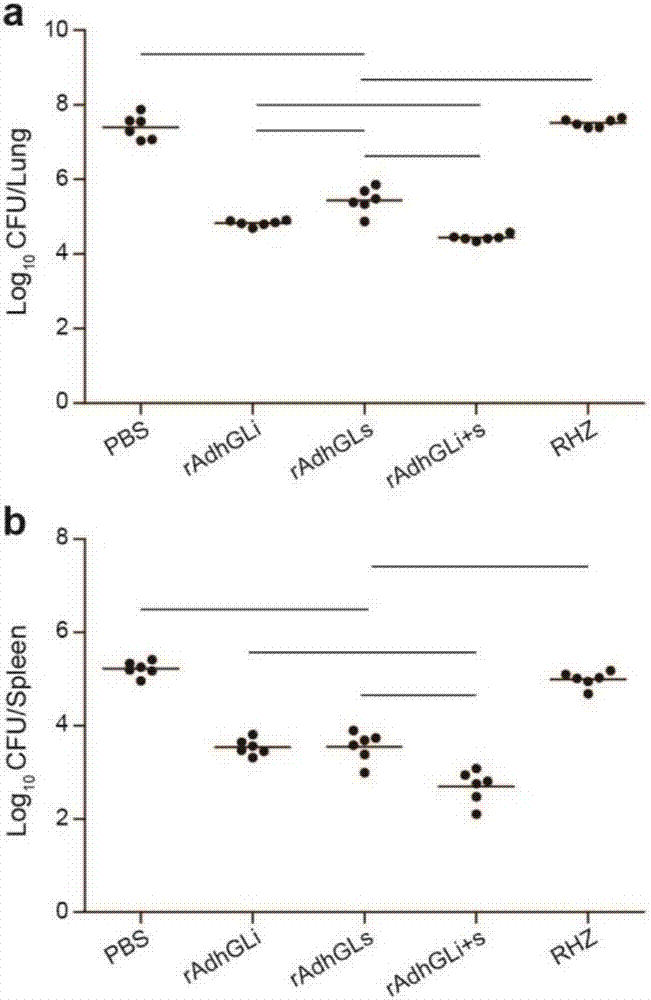
A kind of tuberculosis gene drug and preparation method thereof
A gene drug and tuberculosis technology, applied in the field of biomedicine, can solve problems such as diseases and curative effects that need to be improved, and achieve the effect of improving safety performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] The TB gene medicine provided by the invention, its preparation method comprises the following steps:
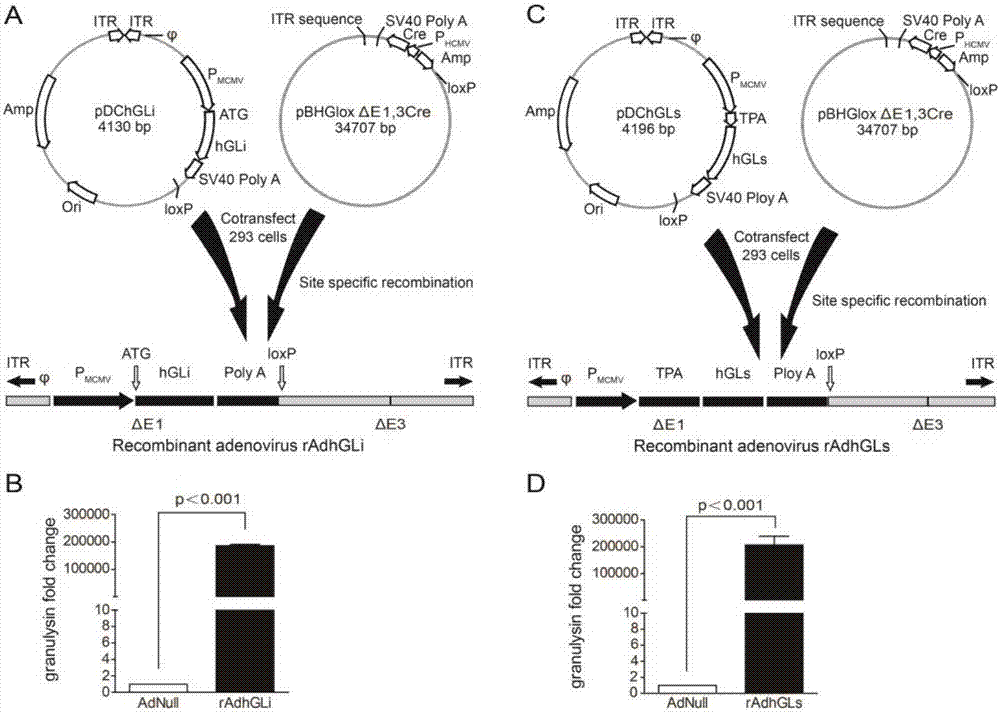
[0055] Step 1, integrating the first sequence expressing intracellular granulysin protein and the second sequence expressing extracellular granulysin protein into the shuttle plasmid to form the intracellular granulysin gene shuttle plasmid (pDChGLi) and the extracellular Granlysin gene shuttle plasmids (pDChGLs);
[0056] The first sequence is preferably:
[0057] (1) the gene sequence shown by SEQ ID No.1; or
[0058] or
[0059] (3) The amino acid sequence defined by SEQ ID No.1 is increased, deleted or replaced by one or more amino acids, and the expression product has a gene sequence with the same activity as the protein expressed by the sequence SEQ ID No.1.
[0060] The second sequence is preferably:
[0061] A, the gene sequence shown by SEQ ID No.2; or
[0062] B. An amino acid sequence with an amino acid sequence homology defined by the sequence SEQ ID ...
Embodiment 1
[0072] Example 1 Packaging and purification of intracellular granulysin recombinant adenovirus (rAdhGLi) and extracellular granulysin recombinant adenovirus (rAdhGLs)
[0073] 1) According to conventional molecular cloning techniques, the gene sequences of SEQ ID No.1 and SEQ ID No.2 were respectively cloned into the adenovirus-Escherichia coli shuttle vector pDC316 to obtain recombinant plasmids pDChGLi and pDChGLs, which were confirmed by enzyme digestion and sequencing.
[0074] 2) HEK293 cells were co-transfected with plasmid pDChGLi (or pDChGLs) and backbone plasmid pBHGloxΔE1,3Cre (1:1) to package recombinant virus rAdhGLi (or rAdhGLs).
[0075] (1) Inoculate HEK293 cells in good growth state in a six-well plate, 5×10 per well 5 Cells were cultured in a 37°C, 5% CO2 cell incubator for 18-24 hours, so that the confluence of the cells reached about 90%. The culture medium was discarded, and 1 mL of complete DMEM medium without antibiotics was added to each well.
[0076]...
Embodiment 2
[0105] Example 2 RT-qPCR confirmed the expression of granulysin mRNA level after rAdhGLi or rAdhGLs infected cells
[0106] 1) AdNull, rAdhGLi and rAdhGLs were infected with HEK293 cells respectively
[0107] (1) HEK293T cells in a good state of trypsinization were resuspended by adding DMEM complete medium and counted at 5×10 5 The total number of cells was inoculated into each well of a six-well plate, supplemented with DMEM complete medium to 2ml, and placed in a 37°C CO2 incubator for 18-24h.
[0108] (2) Discard the old medium in each well, add 2ml of DMEM complete medium without antibiotics, and infect HEK293T with AdNull, rAdhGLi and rAdhGLs at an infection rate of MOI of 10, and culture for 72 hours.
[0109] (3) Collect the cell suspension with sterile 1.5ml EP, centrifuge at 1000rpm / min for 10min, discard the supernatant to collect the cell pellet, wash the cells twice with sterile PBS, discard the supernatant by centrifugation, add 1ml Trizol to each EP tube Repea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com