Method for detecting toxicity of cefazedone sodium impurities and intermediates
A technique for detecting the toxicity of cefazedone sodium, which is applied in measuring devices, material inspection products, testing pharmaceutical preparations, etc., can solve the problem of unclear and different cefazedone sodium impurity detection methods, unfavorable production or storage of cefazedone sodium and other issues to achieve the effect of safe and reliable medication and guaranteed safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
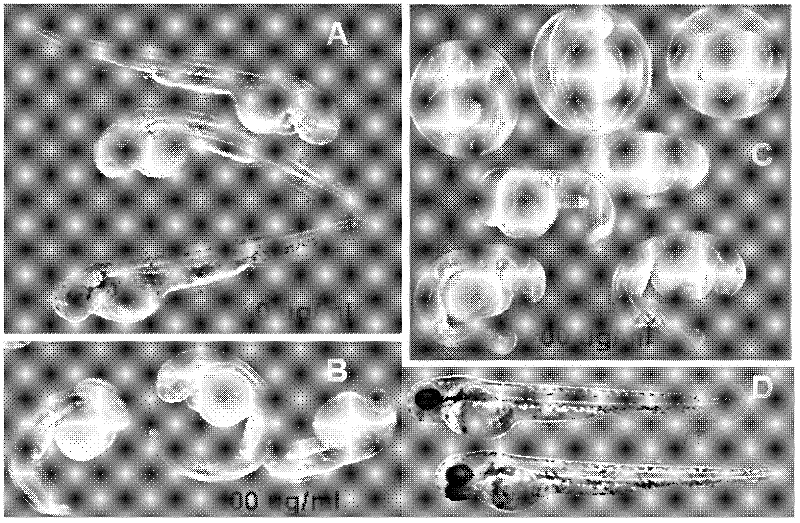
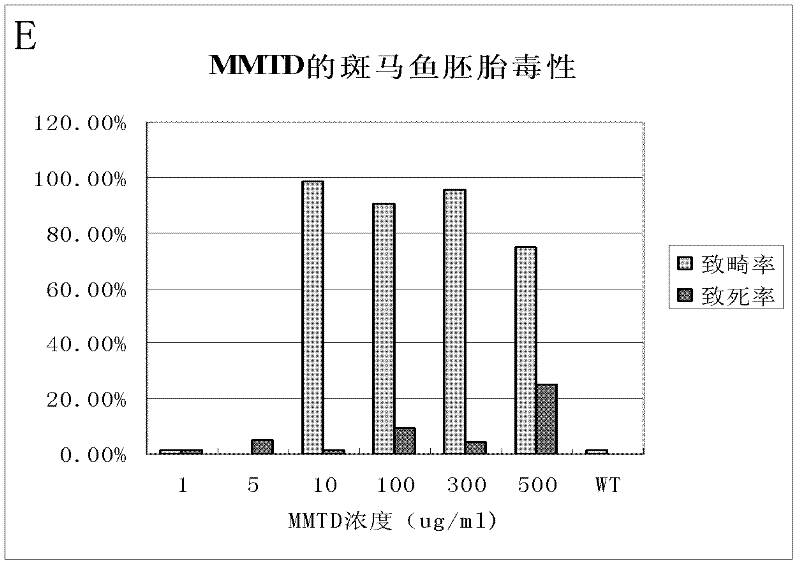
[0041] Below in conjunction with embodiment, the present invention is further described, and following embodiment is illustrative, not restrictive, can not limit protection scope of the present invention with following embodiment.
[0042] The practical artificial seawater of the present invention is commercially available artificial seawater, and there is no special requirement.
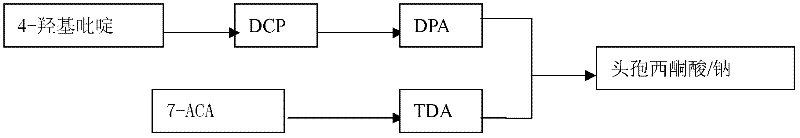
[0043] A kind of preparation method of cefazedone sodium, operation route is as follows:
[0044] first step:
[0045]
[0046] Step two:
[0047]
[0048]
[0049] third step:
[0050]
[0051] 2. Process route diagram and intermediate structure formula:
[0052] 1, the synthesis process flow chart of cefazedone sodium is as figure 1 shown.
[0053] 2. The structural formula of each intermediate
[0054] Table 1 Known impurities of cefoxizone
[0055]
[0056] 3. Analysis of impurities in cefazedone sodium (gradient elution system)
[0057] Gradient elution refers to the elut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com