Method for synthesizing radionuclide-labeled compounds using exchange resins
A radionuclide and resin synthesis technology, applied in the field of computer programs, can solve problems such as unstable yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
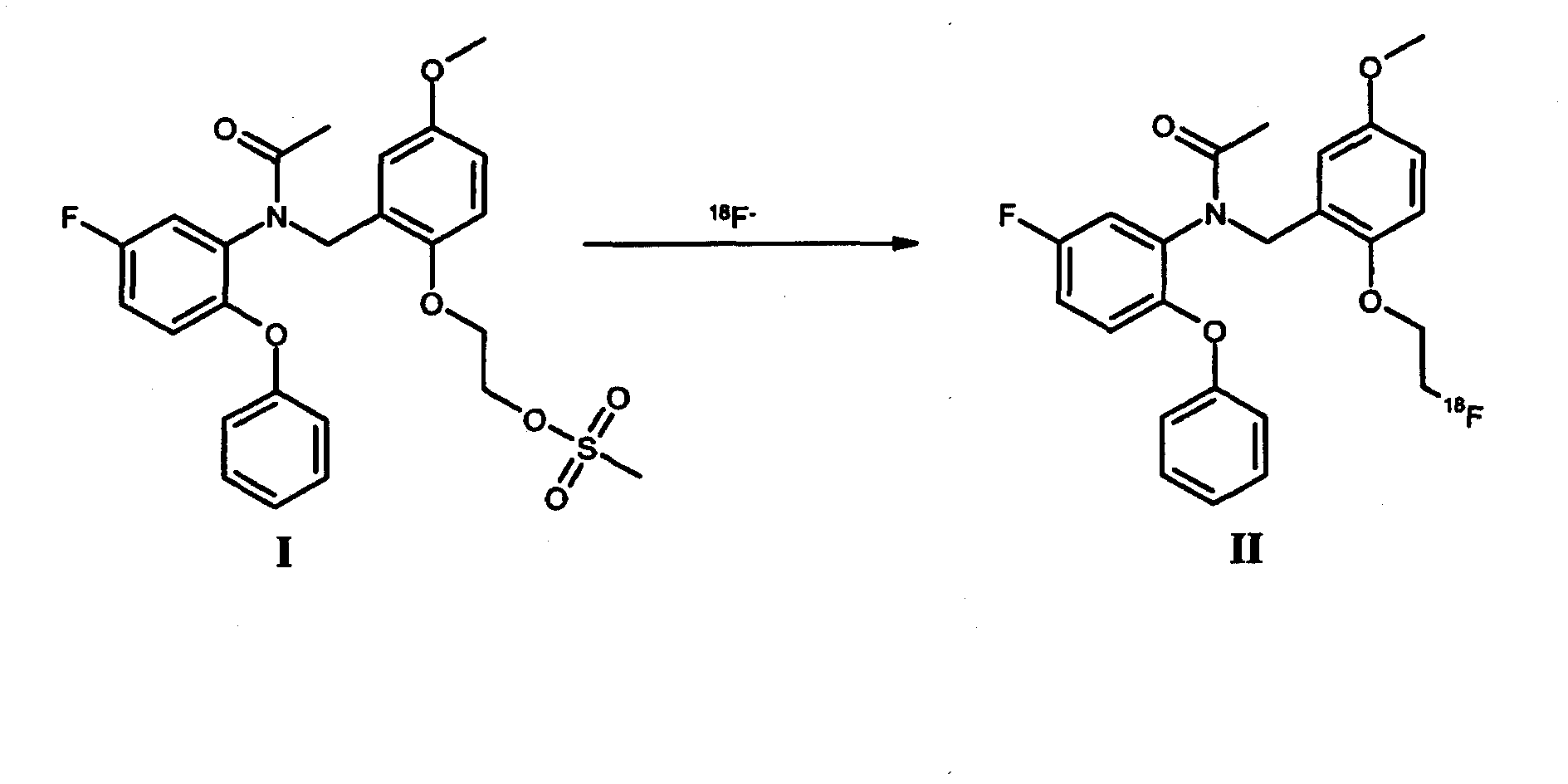
[0103] As radionuclide-labeled compounds [ 18 Synthesis of F]-FEDAA
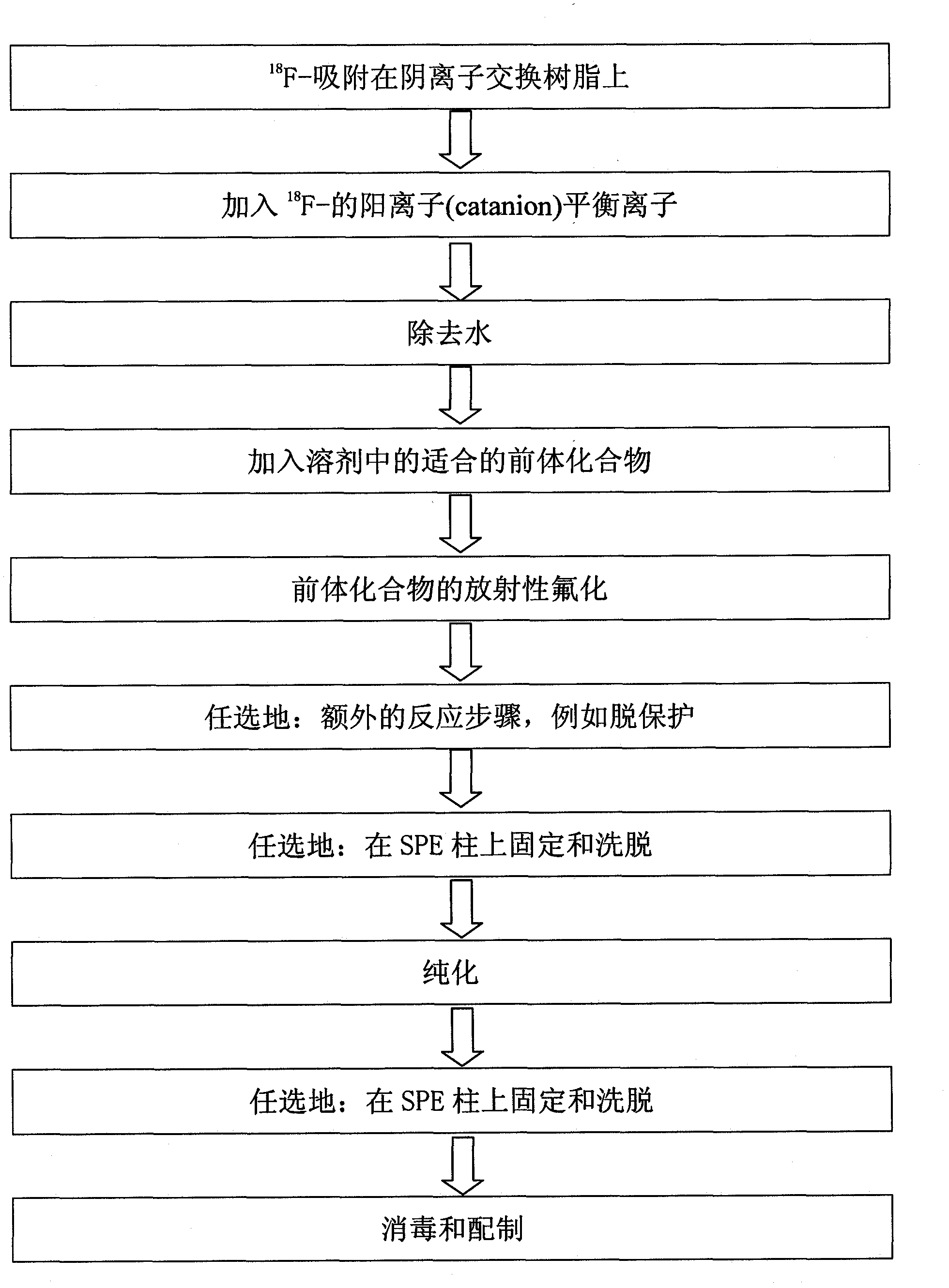
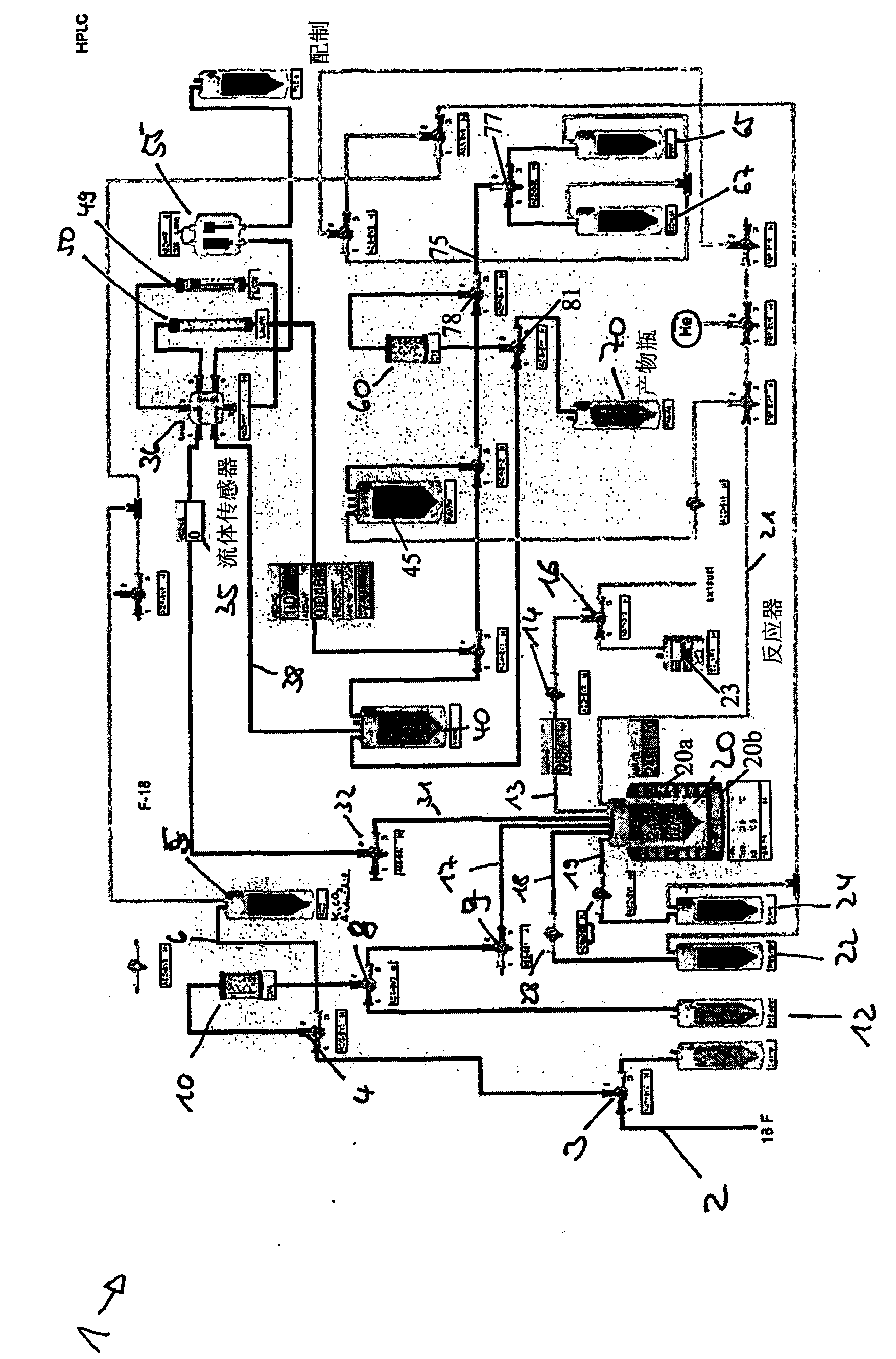
[0104] use image 3 Synthesis of the setup for automated radiolabeling (here radiofluorination) shown in [ 18 F] - FEDAA.
[0105] will 5GBq[ 18 F] Trapped on the first solid-phase extraction resin in the form of a QMA-column with 0.5 mol / l K 2 CO 3 The solution was pretreated and washed with water. Subsequently, with K 222 / K 2 CO 3 solution (dissolved in 0.2ml H 2 1.0 mg K in O and 0.8 ml acetonitrile (ACN) 2 CO 3 , 5.0 mg K 222 )Will[ 18 F] Elution into a reactor preheated to 60°C. Elution was performed in pulsed mode by repeatedly closing and reopening the input tubing with the valve (ACG-SV1) at a 5 second cycle.
[0106] The solvent was evaporated by heating to 110 °C under weak vacuum for 10 min with the aid of a gentle stream of dry nitrogen. dry[ 18 F] KF / K 2.2.2., then 2 mg of the precursor dissolved in DMF (600 μl) were added and heated at a reaction temperature of 120°C. After 5 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com