A kind of doxorubicin and annexin V conjugate, its preparation method and application
An annexin and doxorubicin technology, which is applied to the conjugate of doxorubicin and annexin V and the field of preparation thereof, can solve the problems of large side effects of the drug, poor tumor targeting of doxorubicin, and achieve enhanced anti-tumor effects. Tumor activity, inhibition of tumor angiogenesis and tumor growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
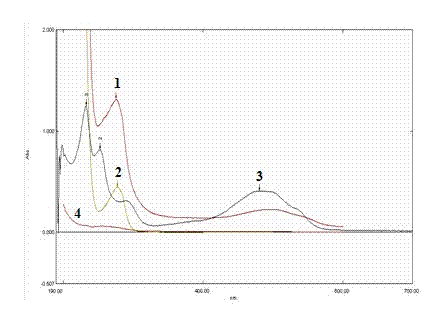
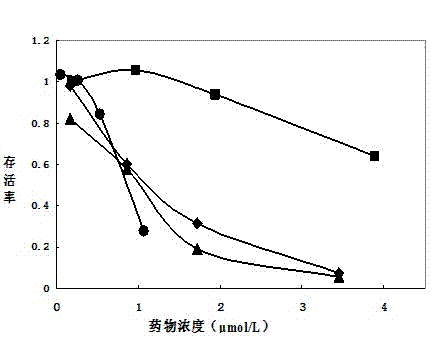
[0023] 1. Activate the carboxyl group on the surface of Annexin V: Dissolve 2 mg of Annexin V freeze-dried powder in 2 ml of 0.05 mM 2-(N-morpholino)ethanesulfonic acid (MES), 0.5 M NaCl, pH=6.0 solution, adjust the concentration to 1 mg / ml, add 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC) to make the final concentration 6 mM. Add N-hydroxysulfosuccinimide (Sulfo-NHS) to a final concentration of 5 mM, mix well and react at room temperature for 15 minutes, then add 2.8 μl 2-mercaptoethanol to a concentration of 20 mM, and react for 10 minutes , to neutralize unreacted EDC.
[0024] 2. Coupling of activated Annexin V with doxorubicin: Use an ultrafiltration tube with a molecular weight cut-off of 10KD to centrifuge and concentrate the intermediate product solution to minimize the volume of the solution to reduce the impact on the pH value of the coupling reaction buffer. Add 3 mg of doxorubicin hydrochloride to 0.1M sodium phosphate solution (pH=7.5) to for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com