Process for preparing aromatic iodine compounds
一种芳族化合物、化合物的技术,应用在制备碘化芳族化合物领域,能够解决无法充分提高反应物进料流量、二碘代化合物产率下降等问题,达到稳定控制、稳定反应温度、延长使用周期的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
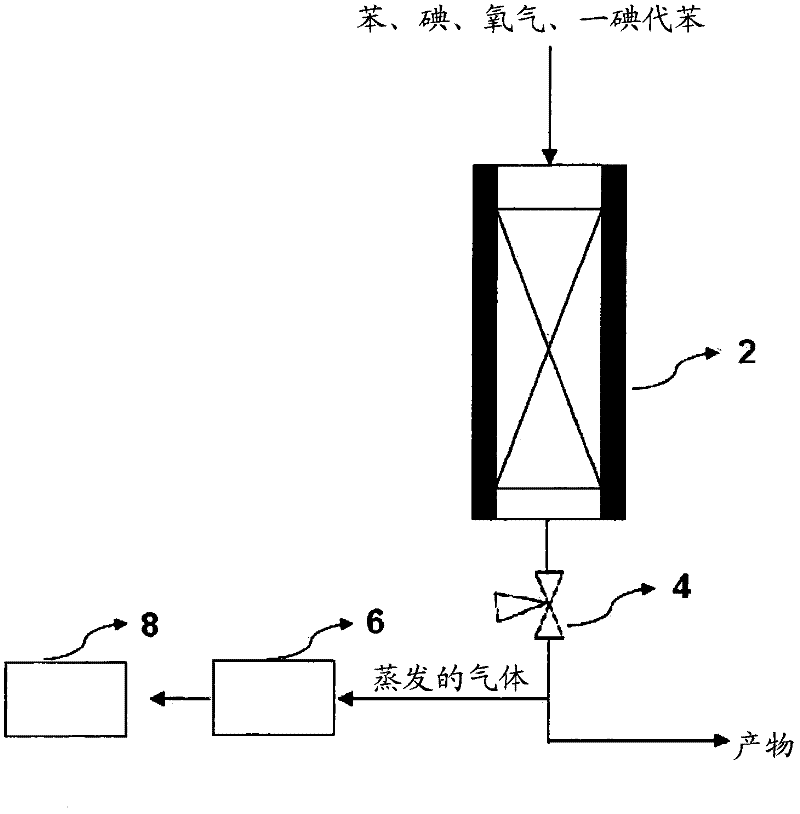
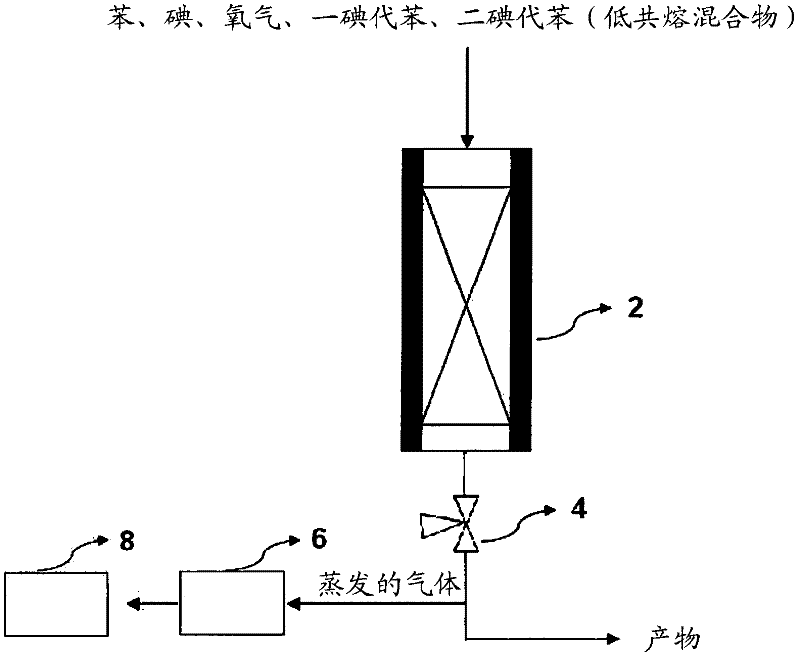
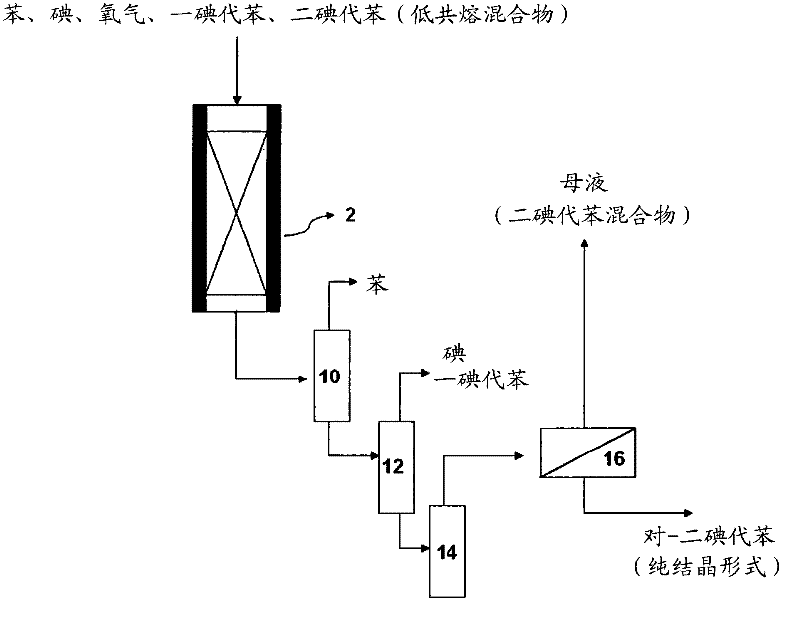
[0072] use as figure 2 The equipment shown, with the same conditions of comparative example 1, add benzene (102.4g / hr), iodine (66.9g / hr) and one iodobenzene (483.8g / hr) in the presence of Na-13X zeolite catalyst to carry out iodine reaction. In addition, diiodobenzene (65.9 g / hr) was also added, based on the total feed material (Feed), wherein the composition of diiodobenzene was 9% (wt%). At this time, the molar ratio of non-halogenated aromatic compound / iodine added was 1.93. The diiodobenzene used in this paper is not a single material, but a liquid diiodobenzene obtained by crystallization and solid / liquid separation of the three isomers (para, ortho, meta) of diiodobenzene Mixture (para, ortho, meta).
[0073] also, Figure 4 Shown is a temperature profile according to the length of the reactor measured at the center of the reactor in the same manner as in Comparative Example 1. Table 1 shows the reaction conditions and the data of the reaction products.
[0074] In...
Embodiment 2
[0076] use as figure 2 The equipment shown, with the same conditions of comparative example 1, add benzene (98.1g / hr), iodine (47.8g / hr) and one iodobenzene (458.7g / hr) in the presence of Na-13X zeolite catalyst to carry out iodine reaction. In addition, diiodobenzene (115.1 g / hr) was added, based on the total feed material (Feed), wherein the composition of diiodobenzene was 16% (wt%). At this time, the molar ratio of non-halogenated aromatic compound / iodine added was 1.93.
[0077] also, Figure 4 Shown is a temperature profile according to the length of the reactor measured at the center of the reactor in the same manner as in Comparative Example 1. Table 1 shows the reaction conditions and the data of the reaction products.
[0078] In particular, all regions of the reactor are maintained at a nearly constant temperature. The temperature in the upper part of the reactor is maintained below about 300°C, which is close to the desired temperature. The reaction temperat...
Embodiment 3
[0080] use as figure 2 The equipment shown, with the same conditions of comparative example 1, add benzene (91.3g / hr), iodine (47.87g / hr) and one iodobenzene (422.7g / hr) in the presence of Na-13X zeolite catalyst to carry out iodine reaction. In addition, diiodobenzene (162.2 g / hr) was added, based on the total feed material (Feed), wherein the composition of diiodobenzene was 22% (wt%). At this time, the molar ratio of non-halogenated aromatic compound / iodine added was 1.93.
[0081] also, Figure 4 Shown is a temperature profile according to the length of the reactor measured at the center of the reactor in the same manner as in Comparative Example 1. Table 1 shows the reaction conditions and the data of the reaction products.
[0082] In particular, all regions of the reactor are maintained at a nearly constant temperature. The temperature in the upper part of the reactor is maintained below about 300°C, which is close to the desired temperature. The reaction tempera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com