Application of ErbB receptor stimulant to preparation of medicament for treating epilepsy
A receptor agonist and epilepsy technology, which is applied in the application field of ErbB receptor agonists in the preparation of drugs for the treatment of epilepsy, and can solve the problems of lack of kinase activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
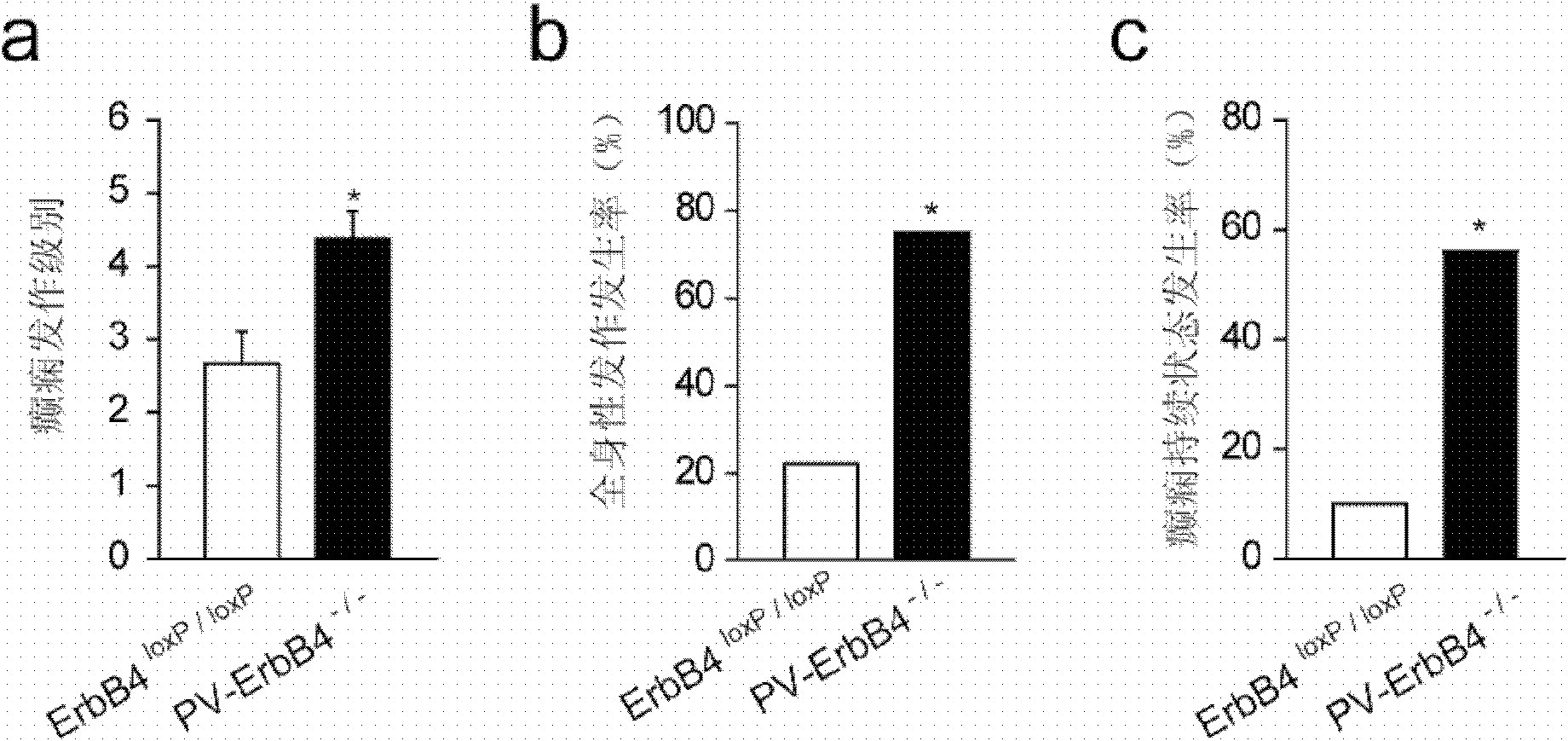
[0024] Example 1: Mice specifically knocking out ErbB4 receptors on PV interneurons are more prone to epilepsy
[0025] (1) Experimental materials:
[0026] Experimental animal: male PV-Cre-ErbB4 LoxP / LoxP Mice (ErbB4 receptor knockout mice) and littermate control ErbB4 LoxP / LoxP Mice (normal mice containing ErbB4 receptors), 18 each, eight weeks old, weighing 24±2 grams, were raised in the same environment, with free intake of food and water; 12 hours of light were given every day. Behavioral experiments were conducted between 12:00 and 14:00.
[0027] ErbB4 receptor-knockout mice were obtained using the widely used Cre-LoxP recombinase system in the world. The system contains two components: ① a 34bp DNA sequence, containing two 13bp inverted repeat sequences and an 8bp core sequence, the sequence is as follows: 5'-ATAACTTCGTATA-ATGTATGC-TATACGAAGTTAT-3'. This 34bp sequence is the site recognized by the recombinase, known as the loxP site; ②Cre recombinase (cyclizationre...
Embodiment 2
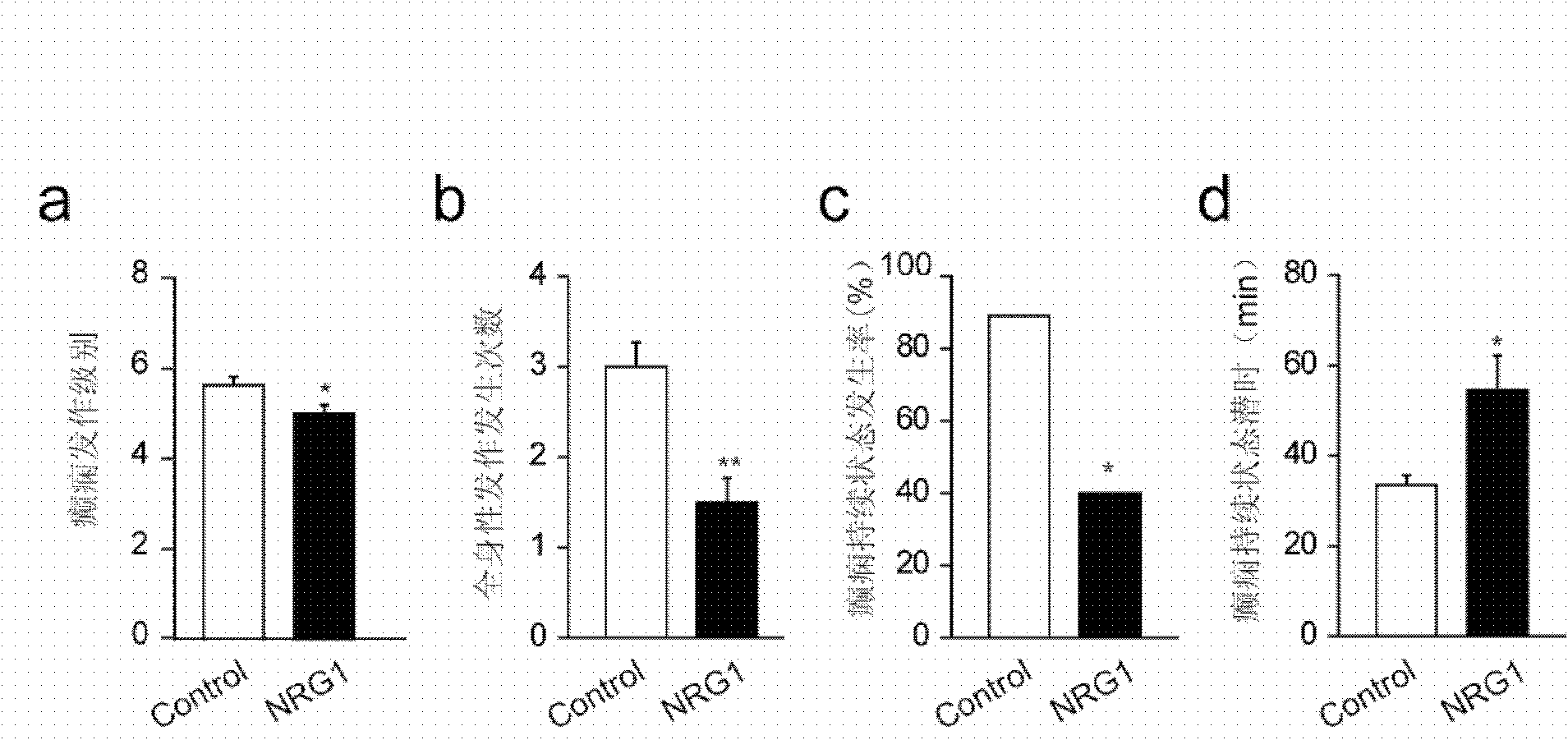
[0038] Example 2: Exogenous intraventricular injection of NRG1 can inhibit epileptic seizures
[0039] (1) Experimental materials:
[0040] Experimental animal: male ErbB4 LoxP / LoxP A total of 20 mice, eight weeks old, weighing 24+2 grams, were raised in the same environment, with free intake of food and water; 12 hours of light were given every day. Behavioral experiments were conducted between 12:00 and 14:00.
[0041] Reagents: PTZ, pilocarpine were purchased from Sigma, USA; Isoflurane (isoflurane) was purchased from Hebei Jiupai Pharmaceutical Company;
[0042] Instrument; stereotaxic apparatus, model 512600, manufactured by Stoelting Company in the United States; anesthesia machine.
[0043] (2) Experimental method:
[0044] According to the previous results, it can be hypothesized that drugs stimulating the NRG1-ErbB4 axis may have antiepileptic effects. Continuous isoflurane (2%) inhalation anesthesia, the mice were fixed under the stereotaxic instrument, after th...
Embodiment 3
[0049] Example 3: The expression of ErbB4 receptors in the brains of human epileptic patients decreased
[0050] (1) Experimental materials:
[0051] Human brain tissue: postoperative brain tissue of 5 patients with intractable epilepsy, meeting the following criteria: I characteristic electroencephalogram, epileptic seizures after more than two years of drug treatment, 2 to 3 first-line antiepileptic drugs used and reached blood level. drug concentration. II Epilepsy symptoms in line with the 1981 International Organization Against Epilepsy classification criteria. No progressive foci under the guidance of IIICT or MRI. IV No neurologic disorder other than epilepsy V Preoperative evaluation pertaining to position-related epileptiform discharges.
[0052] The brain tissues of the control group met the following criteria: I had no history of nervous system diseases. II In addition to the trauma, there is no other injury that can cause changes in brain structure or function ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com