Alprostadil composite medicine, preparation method thereof, quality controlling method thereof, and purpose thereof
A technology for alprostadil and medicine, which is applied in the field of alprostadil combination medicine and its preparation quality control, and can solve the problems of affecting curative effect, producing side effects, poor stability of preparation quality and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
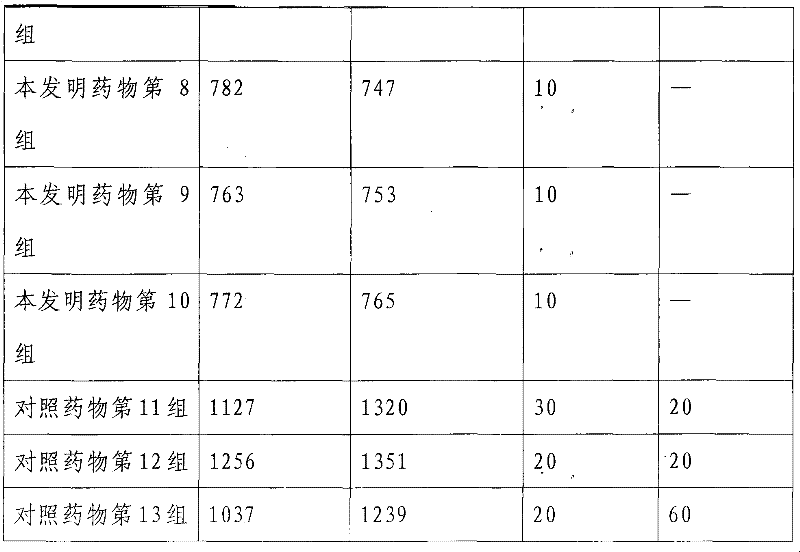
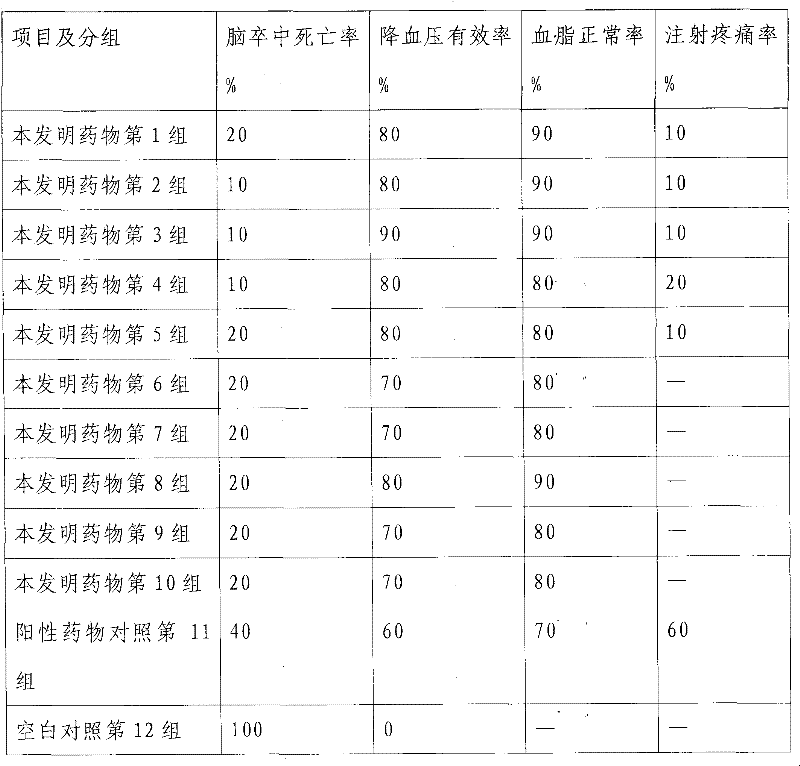
[0050] The weight ratio of each component raw material of a kind of alprostadil combination medicine of the present invention is as follows:
[0051] (1) High-purity alprostadil 0.77
[0052] (2) lysine pirin: sodium salicylate weight ratio is 90-95: 5-10 mixture 210
[0053] (1) Dextran 40 and 2-hydroxypropyl-β-cyclodextrin
[0054] Weight ratio 12-18:1 mixture mixture 500
[0055] (4) Disodium dimercapto 2.00
[0056] (5) Pentetic acid 10
[0057] (6) Activated carbon for injection (activated) 50
[0058] (7) 0.05M phosphate buffer pH value 5.0-7.5 8000
[0059] The present invention also provides the preparation method of described alprostadil combination medicine:
[0060] Operating process and process conditions:
[0061] (1) Vial unscrambling, cleaning, and sterilization
[0062] The 5ml vials are sterilized by the UV lamp from the transfer cabinet and transported into the unscrambling room, where the unscrambled bottles are unscrambled, and the sorted vials are ...
Embodiment 2
[0080] The weight ratio of each component raw material of a kind of alprostadil combination medicine of the present invention is as follows:
[0081] (1) High-purity alprostadil 2.31
[0082] (2) Lysine pirin: sodium salicylate weight ratio is 90-95: 5-10 mixture 300
[0083] (1) Dextran 40 and 2-hydroxypropyl-β-cyclodextrin
[0084] 12-18:1 mixture mixture by weight 580
[0085] (4) Disodium dimercapto 3.00
[0086] (5) Pentetic acid 15
[0087] (6) Activated carbon for injection (activated) 60
[0088] (7) 0.05M phosphate buffer pH value 5.0-7.5 9000
[0089] The present invention provides that the preparation method of the alprostadil combination medicine is the same as that in Example 1.
Embodiment 3
[0091] The weight ratio of each component raw material of a kind of alprostadil combination medicine of the present invention is as follows:
[0092] (1) High-purity alprostadil 1.96
[0093] (2) Lysine pirin: sodium salicylate weight ratio is 90-95: 5-10 mixture 265
[0094] (1) Dextran 40 and 2-hydroxypropyl-β-cyclodextrin
[0095] 12-18:1 mixture mixture by weight 530
[0096] (4) Disodium dimercapto 2.50
[0097] (5) Pentetic acid 12
[0098] (6) Activated carbon for injection (activated) 55
[0099] (7) 0.05M phosphate buffer pH value 5.0-7.5 8600
[0100] The present invention provides that the preparation method of the alprostadil combination medicine is the same as that in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com