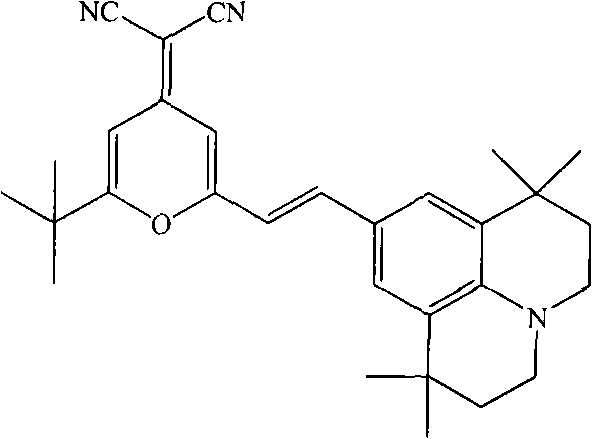
Method for synthesizing 4-(dicyanomethylene)-2-tert-butyl-6-(1,1,7,7-tetramethyljulolidin-4-yl-vinyl)-4H-pyran
A technology of tetramethyl julolidine and dinitrile methylene is applied in the field of synthesis of -4-dinitrile methylene-2-tert-butyl-6-pyran, and can solve the problems of long reaction time and the like , to achieve the effect of good purity and shorten the purification cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
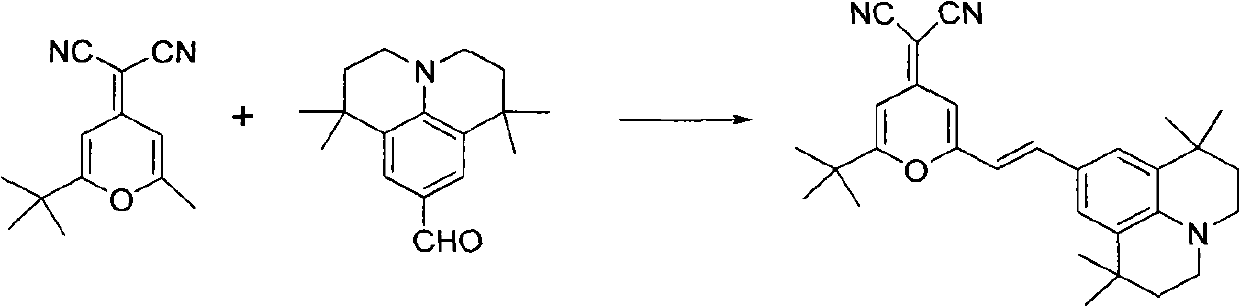
[0020] The present invention is implemented according to the following synthetic route:
[0021]
[0022] In a 100ml round bottom flask equipped with an air condenser, add 2.14g (0.01mol) of 2-methyl-6-tert-butyl-4-dicyanomethenyl-4H-pyran, 9-formyl-1 , 2.57g (0.01mol) of 1,7,7-tetramethyljulonidine, 55ml (1.0mol) of glycerol, and 3.0ml (0.03mol) of piperidine, after making it uniformly mixed at room temperature, added to In the microwave reactor, heat up to the reflux state for 2 minutes, the thin-layer chromatography detects that the reaction is complete, stop the reaction, cool the reaction solution to room temperature, filter, wash the filter cake with 30ml of absolute ethanol, repeat 3 times, dry the filter cake, and obtain (E)-4-Dinitrilemethylene-2-tert-butyl-6-(1,1,7,7-tetramethyljulonidine vinyl)pyran. Then the vacuum is 1.0×10 -3 pa, the temperature is 220°C, after sublimation and purification, 3.85 g of red solid was obtained with a yield of 85% and a purity of...
Embodiment 2
[0027] In a 100ml round bottom flask equipped with an air condenser, add 2.14g (0.01mol) of 2-methyl-6-tert-butyl-4-dicyanomethenyl-4H-pyran, 9-formyl-1 , 3.86g (0.015mol) of 1,7,7-tetramethyljulolidine, 66ml (1.2mol) of glycerol, 3.0ml (0.03mol) of piperidine, after making it uniformly mix at room temperature, add to In the microwave reactor, heat up to the reflux state for 2 minutes, the thin-layer chromatography detects that the reaction is complete, stop the reaction, cool the reaction solution to room temperature, filter, wash the filter cake with 30ml of absolute ethanol, repeat 3 times, dry the filter cake, and obtain (E)-4-Dinitrilemethylene-2-tert-butyl-6-(1,1,7,7-tetramethyljulonidine vinyl)pyran. Then the vacuum is 1.0×10 -3 pa, the temperature is 220° C., after sublimation and purification, 3.86 g of a red solid was obtained, with a yield of 85.2% and a purity of 99.6%.
Embodiment 3
[0029] In a 100ml round bottom flask equipped with an air condenser, add 3.21g (0.015mol) of 2-methyl-6-tert-butyl-4-dicyanomethenyl-4H-pyran, 9-formyl-1 , 2.57g (0.01mol) of 1,7,7-tetramethyljulonidine, 55ml (1.0mol) of glycerol, and 5.0ml (0.05mol) of piperidine, after making it uniformly mixed at room temperature, added to In the microwave reactor, heat up to the reflux state for 2 minutes, the thin-layer chromatography detects that the reaction is complete, stop the reaction, cool the reaction solution to room temperature, filter, wash the filter cake with 30ml of absolute ethanol, repeat 3 times, dry the filter cake, and obtain (E)-4-Dinitrilemethylene-2-tert-butyl-6-(1,1,7,7-tetramethyljulonidine vinyl)pyran. Then the vacuum is 1.0×10 -3 pa, the temperature is 220° C., after sublimation and purification, 3.86 g of a red solid was obtained, with a yield of 85.2% and a purity of 99.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com