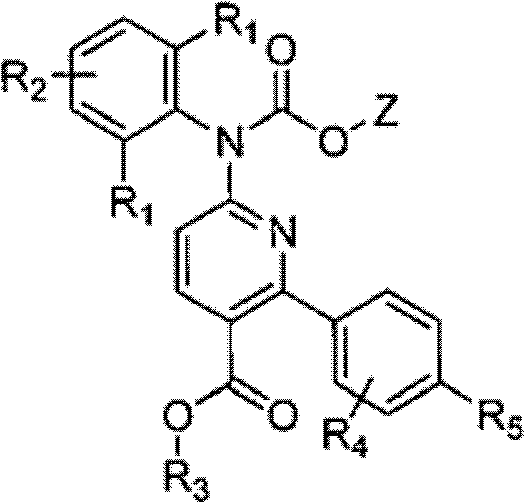
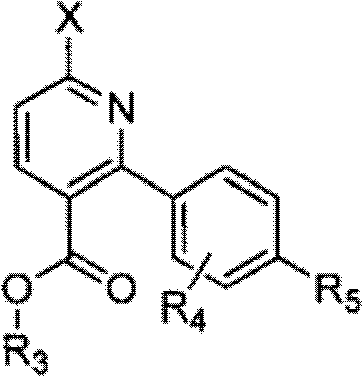
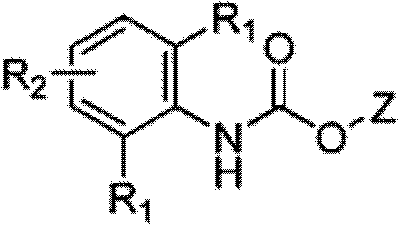
Processes for producing phenyl-6-(1-(phenyl)ureido)nicotinamides)
A nitro and aryl technology, applied in the field of compound preparation, can solve the problems of IL-1β and TNFα block, and achieve the effects of reducing the number of reactions, mild reaction conditions and rapid production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0202] Example 1: Preparation of ethyl 6-chloro-2-(2,4-difluorophenyl)nicotinate (5)
[0203]
[0204] Preparation of ethyl 2-(2,4-difluorophenyl)nicotinate (3)
[0205]
[0206] Add Pd(Ph 3 ) 4 (5.0g, 4.33mmol, 0.005eq), sodium carbonate (92.6g, 874mmol, 1.3eq), ethyl 2-chloronicotinate 1 (126.0g, 678mol, 1.0eq), 2,4-difluorophenylboronic acid 2 (125 g, 791 mmol, 1.2 eq), then 0.5 L of toluene and 125 mL of denatured EtOH were added. The reaction system was stirred vigorously under N 2 Heat to 82° C. overnight under atmosphere (reaction complete by HPLC and TLC). The reaction system was cooled to room temperature, The mixture was pad filtered and the solvent was removed in vacuo at 55°C. The residue was dissolved in EtOAc, washed, dried (MgSO 4 ), and then pass Filter and concentrate. The product was obtained as a yellow solid.
[0207] Preparation of 2-(2,4-difluorophenyl)-3-(ethoxycarbonyl)pyridine 1-oxide (4)
[0208]
[0209] To a nitrogen purged 12 ...
Embodiment 2
[0213] Embodiment 2: Preparation of tert-butyl 2,6-difluorophenylcarbamate (7)
[0214]
[0215] Mix 2,6-difluoroaniline 6 (4.5 mL, 42 mmol, 1.0 equiv) and Boc anhydride (11.1 g, 51 mmol, 1.2 equiv) in THF and add 1 M sodium hexamethyldisilazane to the mixture at room temperature (100 mL, 100 mmol, 2.3 equiv (reaction complete by HPLC). Then 50 mL of brine was added, the solution was concentrated and extracted with EtOAc (2×100 mL). ) to wash the combined organic layers. Then with MgSO 4 The resulting solution was dried, filtered and concentrated to give the title compound 7 as an orange solid which was used in the next step without further purification. 1 H NMR (500.0MHz, CDCl 3 ) 7.18-7.13 (m, 1H), 6.96-6.91 (m, 2H), 6.06 (s, 1H) and 1.52 (s, 9H) ppm
Embodiment 3
[0216] Example 3: Preparation of ethyl 6-(tert-butoxycarbonyl(2,6-difluorophenyl)amino)-2-(2,4-difluorophenyl)nicotinate (8)
[0217]
[0218] A mixture of compound 5 (100.82 g, 0.33 mol, 1.0 eq), compound 7 (101.05 g, 0.44 mol, 1.30 eq) and cesium carbonate (177.12 g, 0.54 mol, 1.60 eq) was suspended in DMSO (250 mL, 2.5 vol ), vigorously stirred at 55-60° C. for 48 h (reaction complete by HPLC). The mixture was cooled to 20-30°C and the base was quenched by cautiously slow addition of 1N HCl(aq) solution (540 mL, 1.60 eq), keeping the internal temperature below 30°C. On cooling, a precipitate formed which was filtered and washed with water (2 x 250 mL, 2 x 2.5 vol). The precipitate was then suspended in absolute ethanol (1000 mL, 10 volumes) and heated to reflux. Reflux was maintained for 30-60 minutes and water (200 mL, 2 volumes) was added to the mixture. The resulting mixture was then reheated to reflux and maintained at reflux for 30 minutes at which time the suspe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com