Application of dendritic cell combined with cytokine-induced killer cell in lung cancer treatment
A cell and lung cancer technology, applied in the application field of dendritic cells combined with cytokine-induced killer cells in the treatment of lung cancer, can solve the problem that there is no data showing the effect of chemotherapy combined with immunotherapy in patients with advanced non-cellular lung cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1, patient and dosing regimen
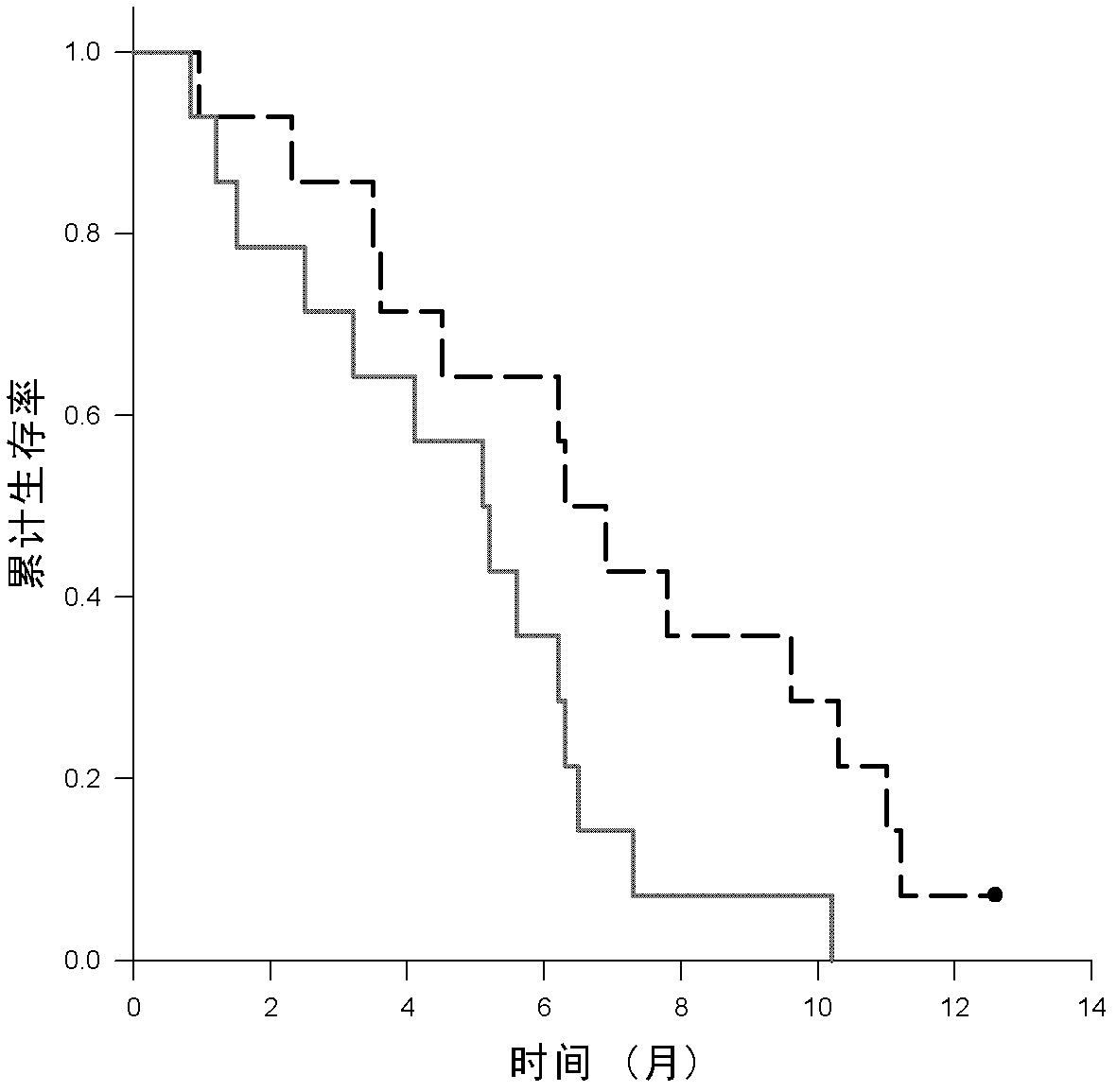
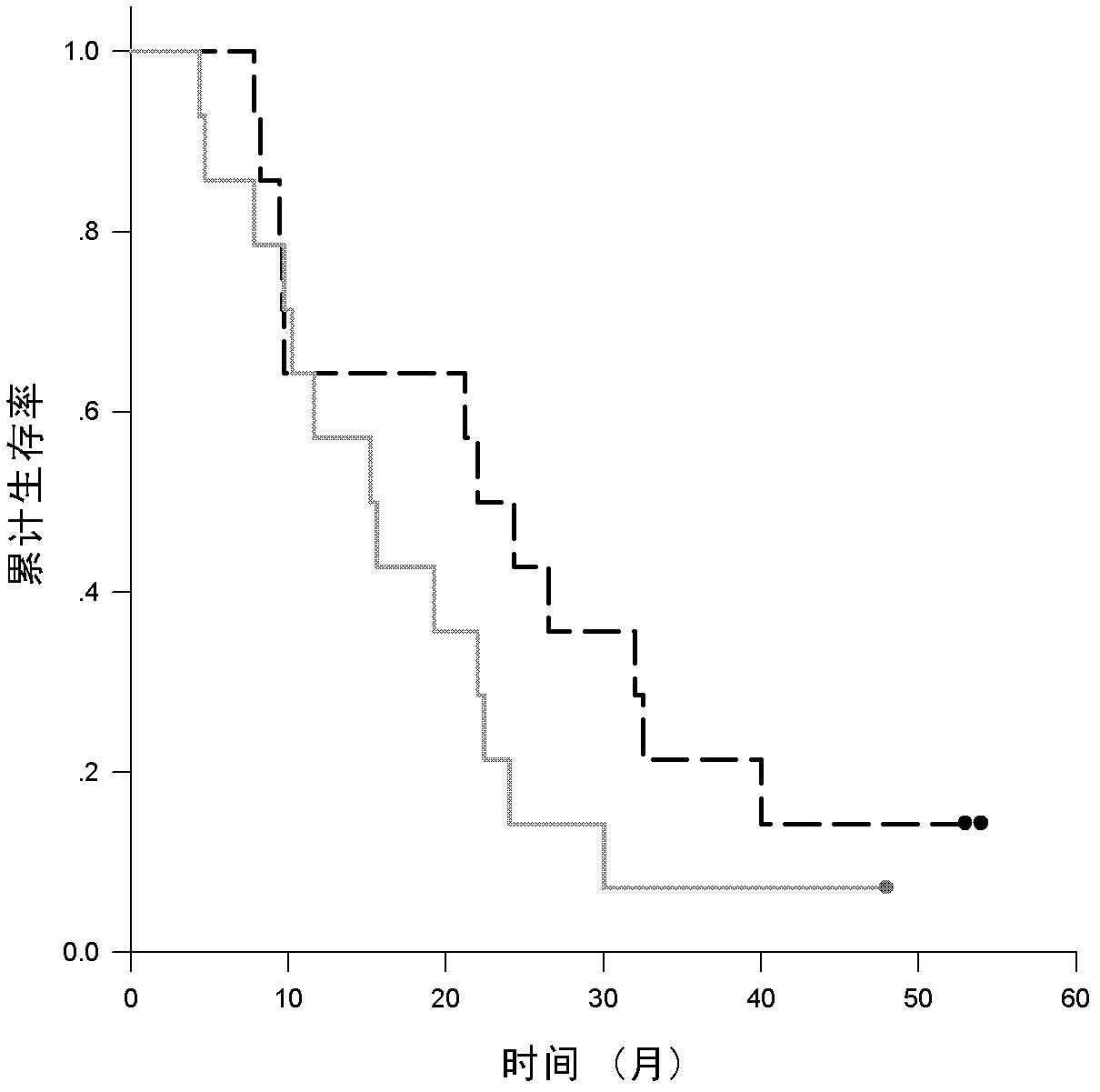
[0054] According to the WHO standard, the patient had histologically confirmed non-small cell lung cancer, and the serum CEA level (measured by conventional chemiluminescent method) was 6-394 ng / ml (normal value: 5 ng / ml). Peripheral blood was drawn from patients who were positive for HLA-A2 confirmed by flow cytometry, and mononuclear cells were isolated. The patient's bone marrow, liver and kidney functions were normal. The patient had no autoimmune diseases, no brain metastases, and no acute or chronic infectious diseases. From August 2004 to October 2005, 28 patients with III B-IV NSCLC were enrolled. Randomly enter the chemotherapy group or immunochemotherapy group. The size of the lesion was evaluated by CT every 4 weeks. Chemiluminescence was used to evaluate the CEA value before and after each treatment. Toxic reactions were in accordance with NCI (2.0) standards. This study was approved by the Ethics Committee of...
Embodiment 2
[0063] Example 2, Preparation of DC and Induction of CIK Production
[0064] Preparation of DC
[0065] In the immunotherapy group, 100 ml of the patient's own peripheral blood was extracted, and the mononuclear cell layer was separated by the Ficoll-Paque density separation method, suspended in the X-VIVO culture medium containing 1% (v / v) autologous serum, and placed in the culture medium. In the dish, adjust the cell concentration to 5×10 6 cells / ml and cultured in a 37°C incubator. After 4 hours, adherent cells (adherent cells) were reserved for DC culture, and non-adherent cells were reserved for CIK cell culture. Adherent cells were suspended with X-VIVO (cell concentration 2×10 6 / ml), add 1000 units (U) / mL of GM-CSF (purchased from Leukomax; Novartis International AG, Basel, Switzerland), 500 units / mL of recombinant IL-4 (purchased from Strathmann Biotec AG, Hannover, Germany) ) to culture DC.
[0066] Peptide sensitization: CEA peptide, the sequence is YLSGANLNL ...
Embodiment 3
[0070] Embodiment 3, administration characteristic
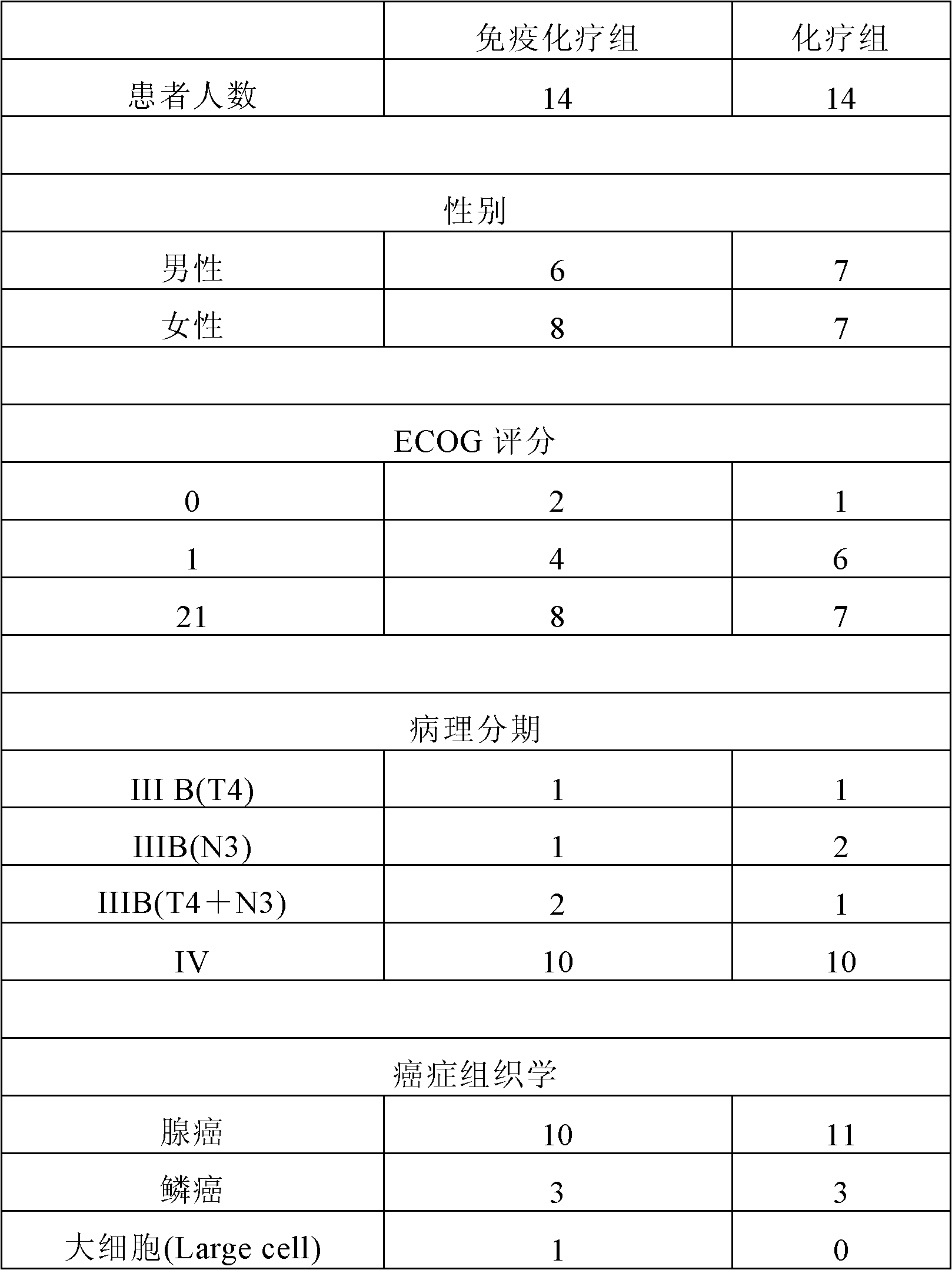
[0071] The characteristics of the patients after the immunochemical group are shown in Table 2. The average amount of injected DC was 8.1±2.5 (10 6 ), the average amount of CIK is 13.3±3.5 (10 8 ) (single injection volume). Serum CEA levels are elevated when they are 25% above baseline. Decline was defined as a 25% decrease from baseline. Otherwise, it is considered stable. In the immunochemotherapy group, CEA levels decreased in 3 patients (21.4%). Stable in 9 patients (64.2%). Grade 1-3 skin toxicity occurred in 9 patients (64.2%), and grade 1-4 non-infectious fever occurred in 10 patients (71.4%).
[0072] Table 2. Characteristics of patients in the immunochemistry group
[0073]
[0074]
[0075]Among them, the grading of skin toxicity follows the National Cancer Institute common toxicity criteria v.2.0 grading system standard.
[0076] Non-infectious fever was graded according to the National Cancer Insti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com