Synthetic method of gamma-aminobutyric acid
A technology of aminobutyric acid and synthesis method, which is applied in the field of synthesis of γ-aminobutyric acid, can solve the problems of low product purity, difficult product purification, and difficult treatment of by-product sodium acetate, and achieve the effect of shortened reaction time and concise process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Put 170 kg of water in a 1000L stainless steel reaction kettle with stirring, heating and devices, then add 170 kg of pyrrolidone (the concentration of the pyrrolidone aqueous solution is 50% by mass), add 204 kg of solid alkali under stirring, and then heat up to the temperature of the kettle The internal temperature is 150°C, and the reaction is continued for 6 hours, the reaction is completed, the reaction solution is obtained, the temperature is lowered, the reaction solution is filtered at 20°C to obtain a γ-aminobutyric acid solution, and then the volume of the γ-aminobutyric acid solution is adjusted at 105°C Concentrate to half, then cool and crystallize the γ-aminobutyric acid solution at 5°C, and dry to obtain 162 kg of γ-aminobutyric acid finished product. The yield was 78.6%, and the main content of γ-aminobutyric acid was 99.81%.
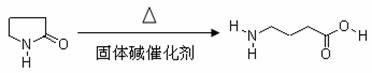
[0022] The reaction equation of the present embodiment is as follows:
[0023]
Embodiment 2
[0025] Put 170kg of water in a 1000L glass-lined reactor with stirring, heating and devices, then add 170KG of pyrrolidone solution, add 340 kg of solid alkali under stirring, then heat up to the temperature in the kettle at 50°C, and continue the reaction for 15 hours to end the reaction. Lower the temperature, filter the reaction solution at 30°C to obtain a γ-aminobutyric acid solution, then concentrate the volume of the γ-aminobutyric acid solution to half at 107°C, then cool the γ-aminobutyric acid solution to crystallize at 4°C , dried to obtain 160.5 kg of gamma-aminobutyric acid finished product. The yield was 77.9%, and the main content of γ-aminobutyric acid was 99.6%. All the other are the same as the description to embodiment 1.
Embodiment 3
[0027] Put 170kg of water in a 1000L stainless steel reaction kettle with stirring, heating and devices, then add 170KG of pyrrolidone solution, add 221kg of solid alkali under stirring, then heat up to the temperature in the kettle at 90°C, and continue the reaction for 12 hours , to end the reaction. Lower the temperature, filter the reaction solution at 25°C to obtain a γ-aminobutyric acid solution, then concentrate the volume of the γ-aminobutyric acid solution to half at 106°C, then cool the γ-aminobutyric acid solution to crystallize at 6°C , dried to obtain 188.6 kg of gamma-aminobutyric acid finished product. The yield is 91.55%, and the main content of γ-aminobutyric acid is 99.6%. All the other are the same as the description to embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com