Pseuoginsenoside-Rh 2 and application to preparation of tumor treating medicine
A technology for simulating ginsenosides and antitumor drugs, applied in the field of traditional Chinese medicine, can solve problems such as different side chain structures, and achieve the effect of obvious curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
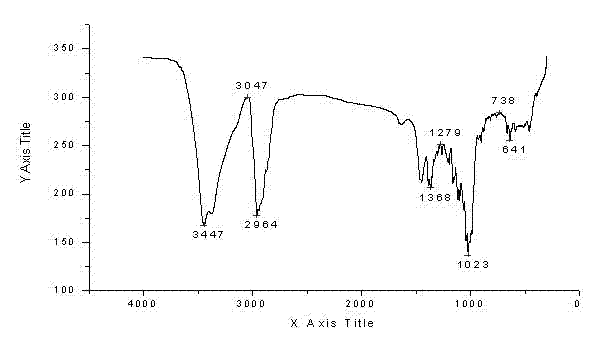
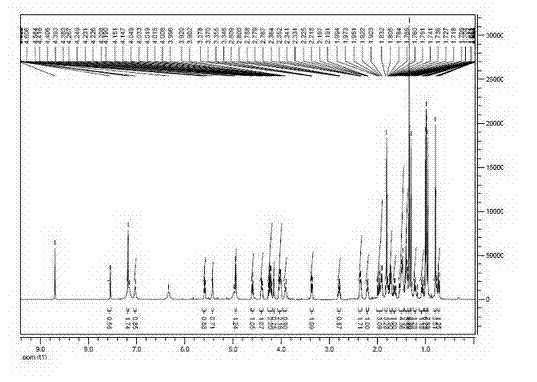
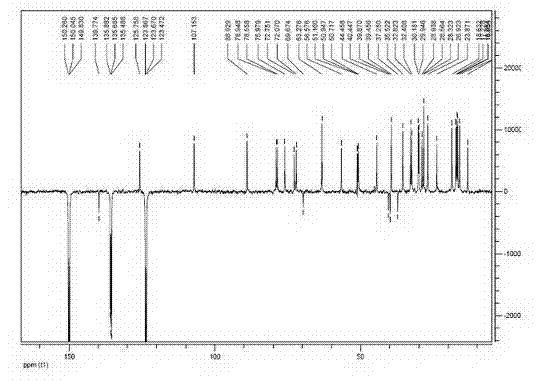
[0068] Preparation and Structure Identification of Pseudoginsenoside-Rh2
[0069] Preparation
[0070] Take 60g of American ginseng stem and leaf diol group saponins and dissolve them in 800mL of 95% ethanol, and then add 800mL of 4% sulfuric acid aqueous solution. After mixing evenly, heat to 90°C and reflux for 5h to terminate the reaction. Adjust pH=6~8 with NaOH. The solvent was recovered, filtered, and the precipitate was dissolved in 95% ethanol, filtered, and evaporated to dryness to obtain 24 g of the converted product.
[0071] Take 24 g of the total conversion product, and use column chromatography silica gel dry packing. With eluent CHCl 3 -MeOH-EtOAc-H 2 O (2:2:4:1, lower layer) was eluted, and the received effluent was detected by thin-layer chromatography, and the corresponding effluents were combined, concentrated to dryness, and then silica gel column chromatography was performed, and CHCl 3 -MeOH-EtOAc-H 2 O (1.5:2:4:1, lower layer) was eluted, and th...
Embodiment 2
[0094] Dissolve 10g of ginsenoside Rb1 in 100mL of methanol, and then add 100mL of 5% aqueous hydrochloric acid. After mixing evenly, heat to 80°C and reflux for 6h to terminate the reaction. Adjust pH=6~8 with NaOH. The solvent was recovered, filtered, and the precipitate was dissolved in 95% ethanol, filtered, and evaporated to dryness to obtain 3.8 g of the converted product.
[0095] Take 3.8 g of the above-mentioned conversion product and pack it into a column with silica gel for column chromatography. With eluent CHCl 3 -MeOH-EtOAc-H 2 O (2:2:4:1, the lower layer) elutes, and the received effluent is detected by thin-layer chromatography, combined with the corresponding effluent, concentrated to dryness, and then silica gel column chromatography, and CHCl 3 -MeOH-EtOAc-H 2 O (1.5:2:4:1, lower layer) was eluted, and the received effluent was detected by thin-layer chromatography, and the corresponding effluents were combined and concentrated to dryness. Component I ...
Embodiment 3
[0097] Take 20g of panaxadiol group saponins and dissolve in 300mL of dioxane, then add 300mL of 5% sulfuric acid aqueous solution. After mixing evenly, heat to 100°C and reflux for 4h to terminate the reaction. Adjust pH=6~8 with NaOH. The solvent was recovered, filtered, and the precipitate was dissolved in 95% ethanol, filtered, and evaporated to dryness to obtain 7.9 g of the converted product.
[0098] Take 7.9 g of the total conversion product, and use column chromatography silica gel dry packing. With eluent CHCl 3 -MeOH-EtOAc-H 2 O (1.5:2.2:4:1, lower layer) was eluted, and the received effluent was detected by thin-layer chromatography, and the corresponding effluents were combined, concentrated to dryness, and then carried out by silica gel column chromatography, and analyzed with CHCl 3 -MeOH-EtOAc-H 2 O (1.5:2:4:1, lower layer) was eluted, and the received effluent was detected by thin-layer chromatography, and the corresponding effluents were combined and con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com