Application of chalcone compounds in preparations of inflammation resisting medicines
A compound and drug technology, applied in the direction of anti-inflammatory agents, drug combinations, active ingredients of ketones, etc., can solve the problem that the pharmacological activity cannot be predicted from the structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The synthesis of embodiment 1 compound
[0027] Dissolve 1 mmol of the corresponding substituted benzaldehyde and substituted acetophenone in 10-20 mL of absolute ethanol, add 5-10 drops of 40% NaOH solution at 5-8°C, react for 5-24 hours at 5-8°C, and detect with TLC The reaction proceeds. After the reaction, 1-2 times the volume of the reaction liquid was added to precipitate a precipitate, which was suction filtered and vacuum-dried overnight to obtain a powder product, which was purified by silica gel column chromatography to obtain compounds with a purity greater than 98%. Among the hundreds of compounds synthesized, the pharmacological activities vary greatly, and the representative compounds and their physicochemical properties are as follows:
[0028] Comparative compound L1H2: (E)-4-(dimethylamino)Chalcone(L1H2).Yellow crystal, 32.4% yield, mp 97.8~98.9℃. 1 H-NMR (CDCl 3 )δ: 8.003(d, J=7.8Hz, 2H, H-2', H-6'), 7.793(d, J=15.6Hz, 1H, H-β), 7.552(d, J=8.4Hz, 2...
Embodiment 2
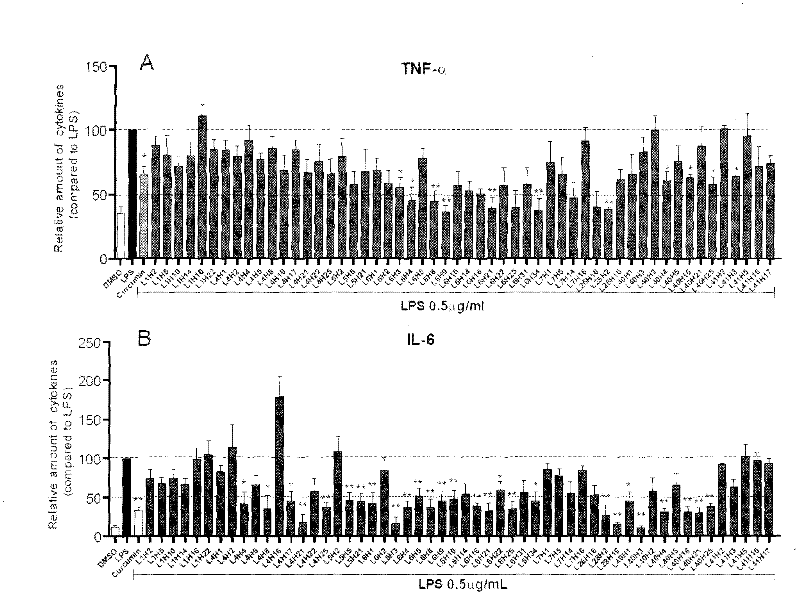
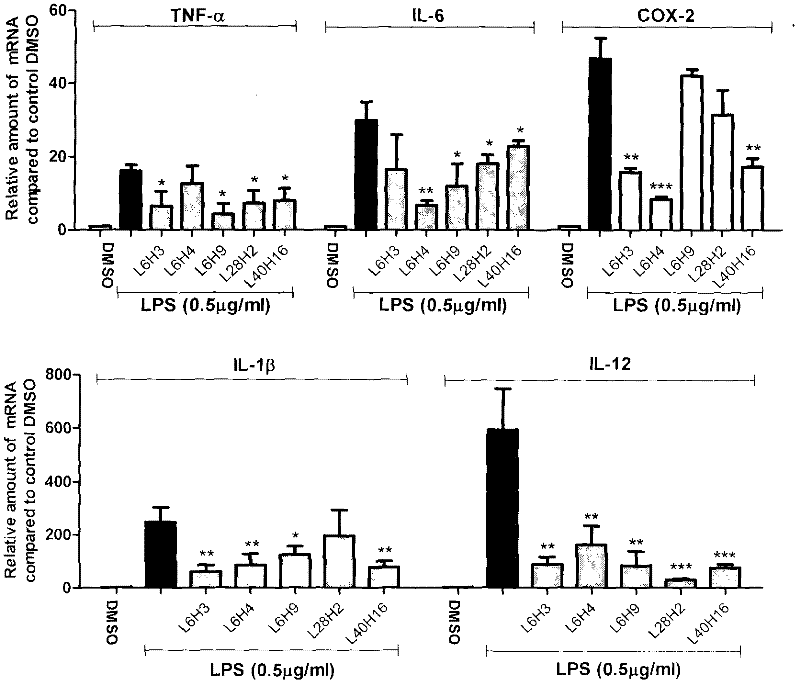
[0063] Inhibition of the compound of Example 2 on the release of inflammatory factors from macrophages stimulated by LPS
[0064] The preliminary anti-inflammatory activity of the compound in vitro was tested by using the compound to inhibit the release of inflammatory factors (TNF-α and IL-6) from RAW 264.7 macrophages stimulated by LPS. The specific method is as follows: 1.2×10 6 RAW 264.7 macrophages were cultured with DMEM medium at 37°C. After 24 hours, the medium was renewed, and the test compound (final concentration was 10 μM) was added for pretreatment for 2 hours, and then treated with 0.5 μg / mL LPS for 22 hours. , collect the culture fluid and use ELISA method to detect the content of TNF-α and IL-6; collect the cells to detect the total protein concentration, and the ELISA results are divided by the corresponding total protein concentration for calibration, and the TNF-α and IL-6 content of the LPS control group The calibration was 100; each compound was tested thr...
Embodiment 3
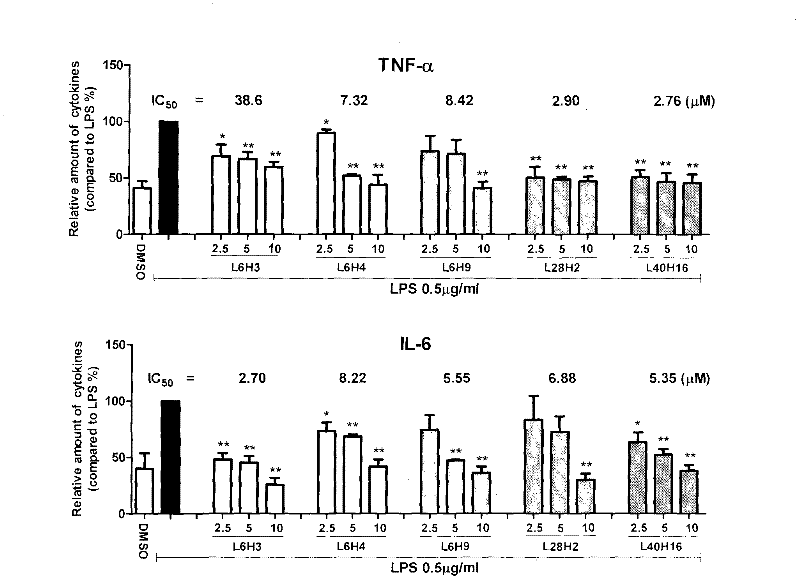
[0065]Example 3 The dose-effect relationship of the active compound inhibiting the release of inflammatory factors from macrophages stimulated by LPS
[0066] The dose-effect relationship of active compounds inhibiting the release of TNF-α and IL-6 from RAW 264.7 macrophages stimulated by LPS was further tested. The method was the same as in Example 2. For experimental data, see figure 2 . The inhibitory activities of the compounds on TNF-α and IL-6 all have a good dose-effect relationship, and the IC 50 ICs of IL-6 inhibitory activity were 38.6, 7.32, 8.42, 2.90, 2.76 μM, respectively 50 2.7, 8.22, 5.55, 6.88, and 5.35 μM, respectively. It can be seen that these compounds have medicinal prospects.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com