Synthesis method of sec-butyl mercaptan
A sec-butyl mercaptan, synthetic method technology, applied in the direction of mercaptan preparation, organic chemistry, etc., can solve the problem of unretrieved sec-butane mercaptan, and achieve the effect of environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
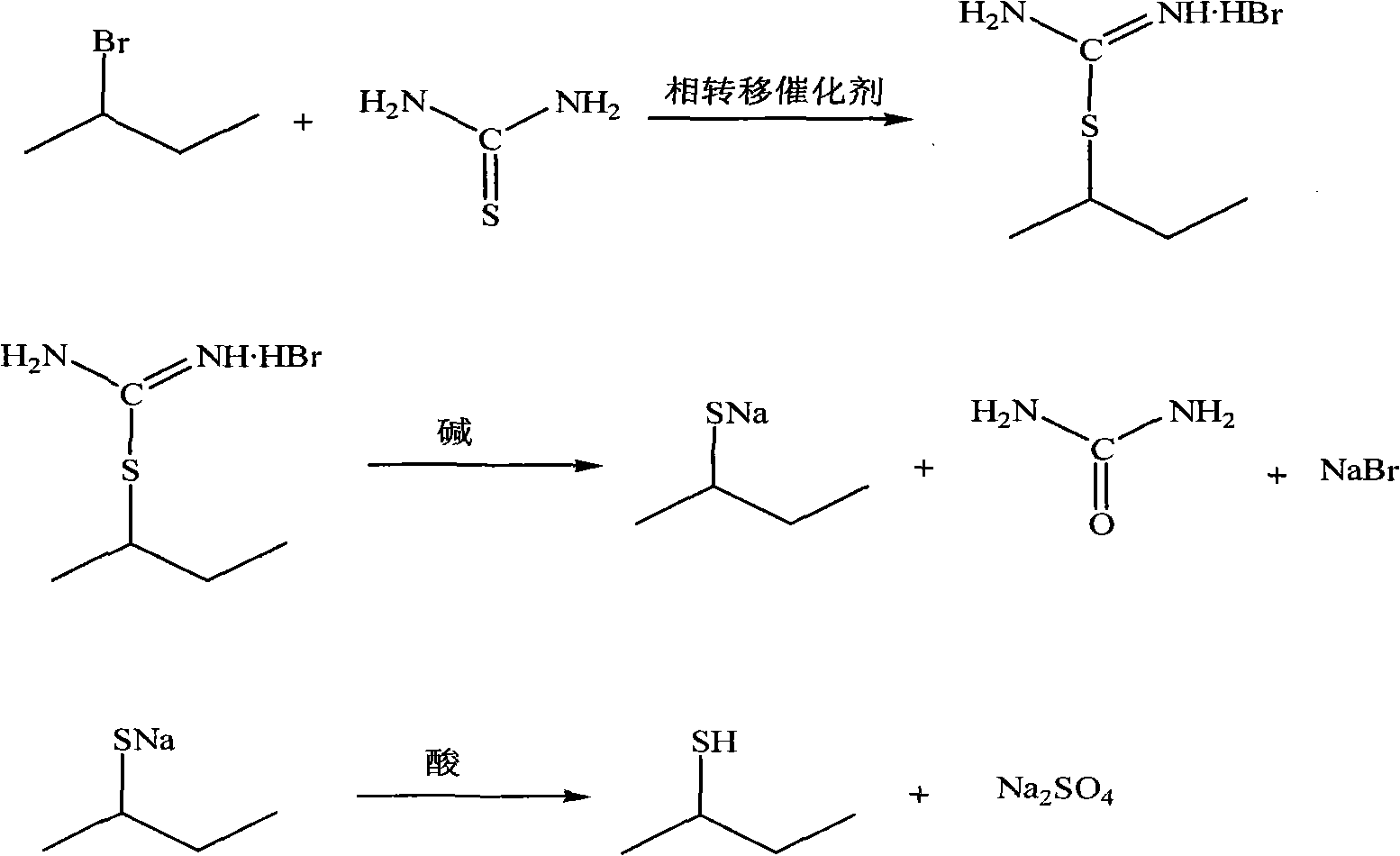
[0015] Add 30ml of water, 7.6g (0.1mol) of thiourea, and 0.2g (0.62mmol) of tetrabutylammonium bromide to the reaction flask, heat up to 50°C under stirring, add 13.7g (0.1mol) of 2-bromobutane, After adding 2-bromobutane, react at 50° C. for 1 hour; the reaction system is heated to 60° C., start to add 12 g (0.3 mol) 20% (weight) NaOH aqueous solution in the reaction flask, and keep 60°C after adding the NaOH aqueous solution. ℃ stirring reaction for 1.5 hours; stop heating, change to distillation apparatus, use 47% (mass) H 2 SO 4 Aqueous solution, adjust the pH of the reaction solution to 1-2, start distillation, the obtained organic layer is dried with anhydrous calcium chloride, filtered, the obtained filtrate is distilled again, and the fraction at 84°C is collected to obtain 7.01g of sec-butylmercaptan. The gas chromatography purity is 99.1%, and the yield is 77.8%.
[0016] Structure Identification:
[0017] Infrared spectrum (potassium bromide): 2923, 2966cm -1 Th...
Embodiment 2
[0021] Add 30ml of water, 7.6g (0.1mol) of thiourea, 0.00685g (0.0212mmol) of tetrabutylammonium bromide to the reaction flask, heat up to 50°C under stirring, add 13.7g (0.1mol) of 2-bromobutane, After adding 2-bromobutane, react at 50°C for 1 hour; heat the reaction system to 60°C, start to add 12g (0.3mol) 20% (weight) NaOH aqueous solution to the flask, and keep 60°C after adding NaOH aqueous solution Stirring reaction 1.5 hours; Stop heating, change distillation unit into, use 47% (mass) H 2 SO 4 Aqueous solution, adjust the pH of the reaction solution to 1 ~ 2, start distillation, the obtained organic layer is dried with anhydrous calcium chloride, filtered, the obtained filtrate is distilled again, and the fraction at 84 ° C is collected to obtain 6.25 g of a colorless liquid, namely sec-butylmercaptan. The gas chromatography purity is 99.0%, and the yield is 68.7%.
Embodiment 3
[0023] Add 30ml of water, 7.6g (0.1mol) of thiourea, and 0.685g (2.12mmol) of tetrabutylammonium bromide to the reaction flask, heat up to 50°C under stirring, add 13.7g (0.1mol) of 2-bromobutane, After adding 2-bromobutane, react at 50°C for 1 hour; heat the reaction system to 60°C, start to add 12g (0.3mol) 20% (weight) NaOH aqueous solution to the flask, and keep 60°C after adding NaOH aqueous solution Stirring reaction 1.5 hours; Stop heating, change distillation unit into, use 47% (mass) H 2 SO 4 Aqueous solution, adjust the pH of the reaction solution to 1-2, start distillation, the obtained organic layer is dried with anhydrous calcium chloride, filtered, the obtained filtrate is distilled again, and the fraction at about 84 ° C is collected to obtain 6.97 g of a colorless liquid. That is, sec-butylmercaptan. The gas chromatography purity is 99.1%, and the yield is 77.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com