Antibacterial protein and its coding gene, expression system, refolding system and application
An antibacterial protein, expression system technology, applied in application, antibacterial drugs, genetic engineering and other directions, can solve the problem that diseases are difficult to control fundamentally
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1. A cloned Penaeus chinensis antibacterial protein gene with the following sequence:
[0034] (1) SEQ ID No 1 information (see Sequence Listing)
[0035] (a) Sequence characteristics:
[0036] *Length: 681 base pairs
[0037] *Type: Nucleic Acid
[0038] *Chain type: Double chain
[0039] *Topological structure: linear
[0040] (b) Molecular type: cDNA
[0041] (c) Assumption: No
[0042] (d) Antonym: No
[0043] (e) Original source: Fenneropena eus chinensis
[0044] (2) SEQ ID No. 2 information (see sequence listing)
[0045] (a) Sequence features
[0046] *Length: 154 amino acids
[0047] *Type: Amino acid
[0048] *Chain type: single chain
[0049] *Topological structure: linear
[0050] (b) Molecular type: protein
[0051] Sequence description: SEQ ID No.1
[0052] 1 tcgtagtgttatcgaggcagctcttatactggtccggtcgttttgcaatacatgtcaaga 60
[0053] 61 gagactacgtggcacacatttaactcaccctccgtacaATGCAGCAGGTACTGTATTCTG 120
[0054] M Q Q V L Y S 7
[0055] 1...
Embodiment 2
[0129] Example 2. In vitro recombinant expression, isolation and purification and biological activity analysis of the Carcinin gene of Penaeus chinensis:
[0130] 1. Construction of recombinant expression vector
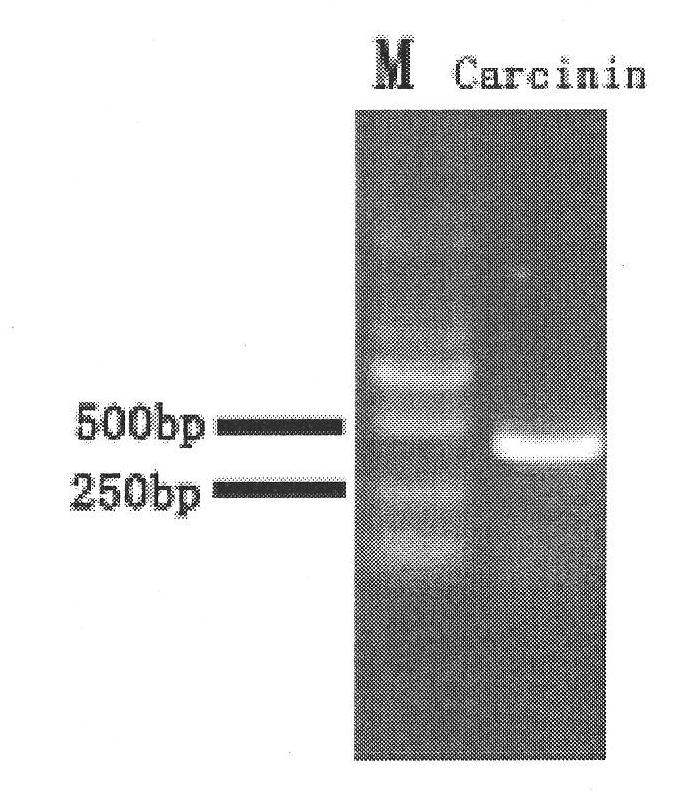
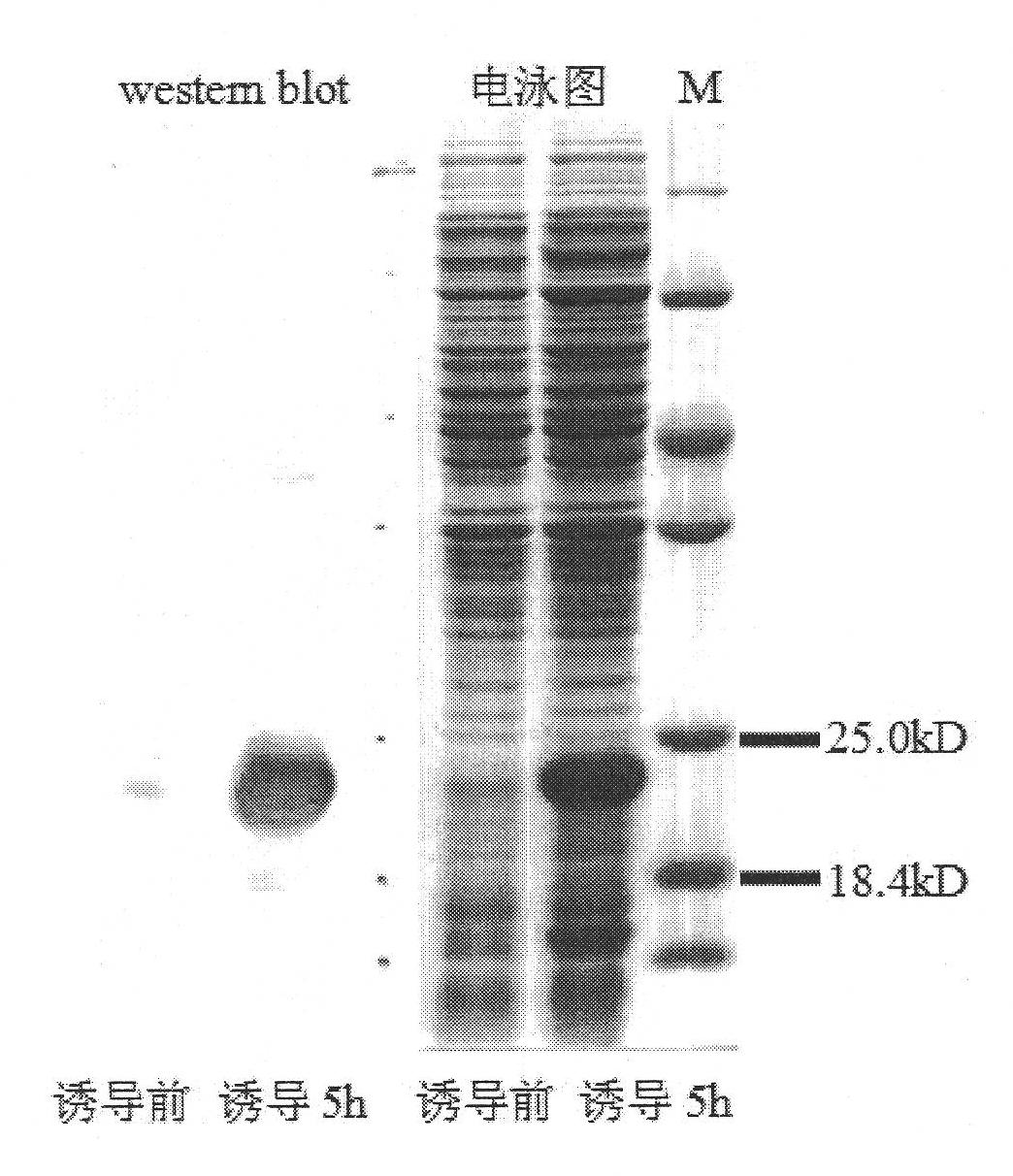
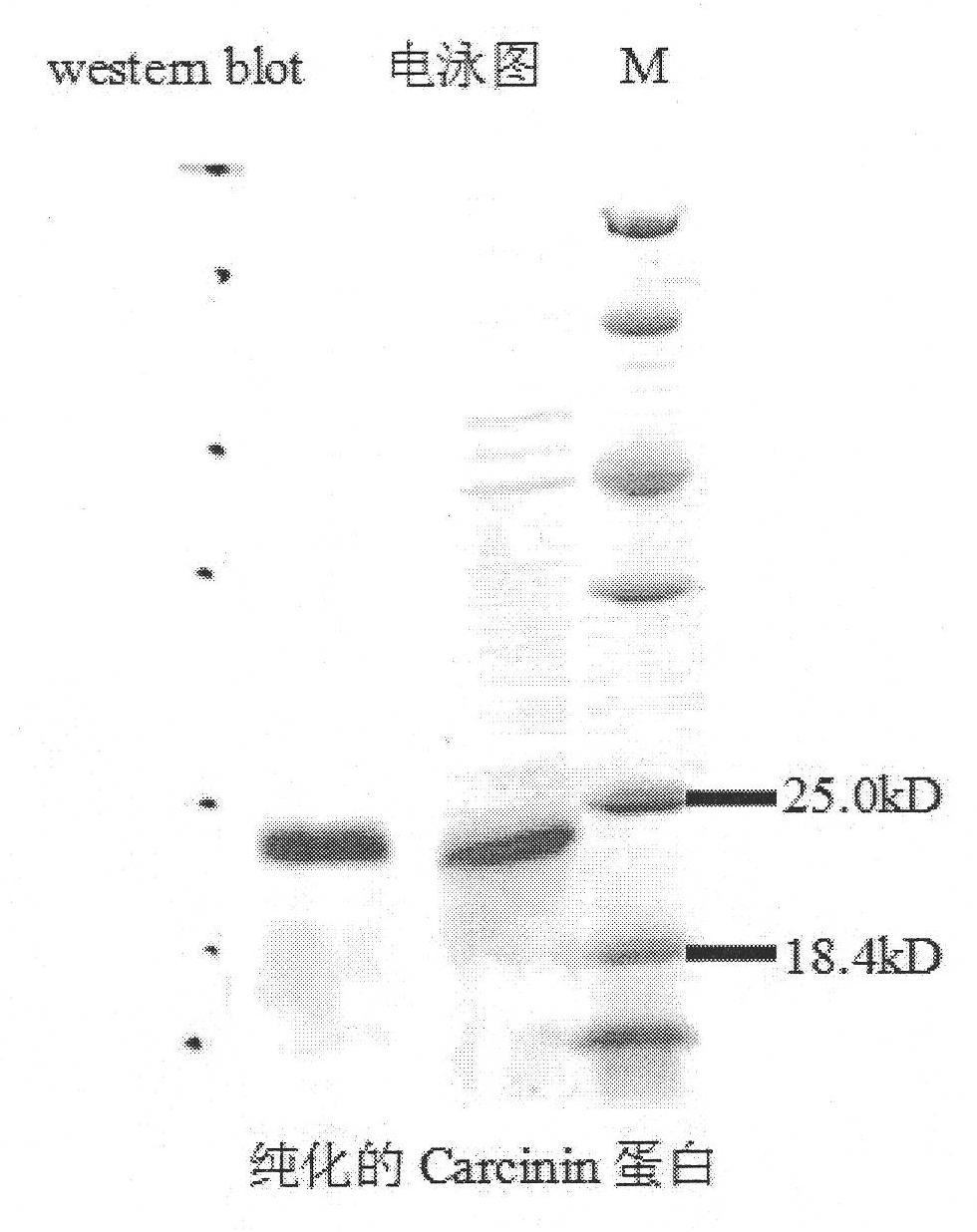
[0131] Submit the gene Carcinin (SEQ ID No.1) to the signal peptide analysis software (http: / / www.cbs.dtu.dk / services / SignalP / ), and the analysis results show that the whole Carcinin protein (SEQ ID No.2) peptide There is a hydrophobic signal peptide at the N-terminus of the chain with a total of 20 amino acids (MQQVLYSALLWACLWNGVRS). The mature Carcinin protein that is secreted outside the cell does not contain a signal peptide. Therefore, a pair of specific primers (FcCar-F: 5'CAT GGA TCC CCGATC TAC GCC ACA GAA 3', 5'CAT GGA TCC CCGATC TAC GCC ACA GAA 3', a pair of specific primers introduced with two restriction sites were designed according to the cloned Penaeus chinensis Carcinin cDNA mature peptide coding sequence. Introduce BamH I restriction site GGA TCC at the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com