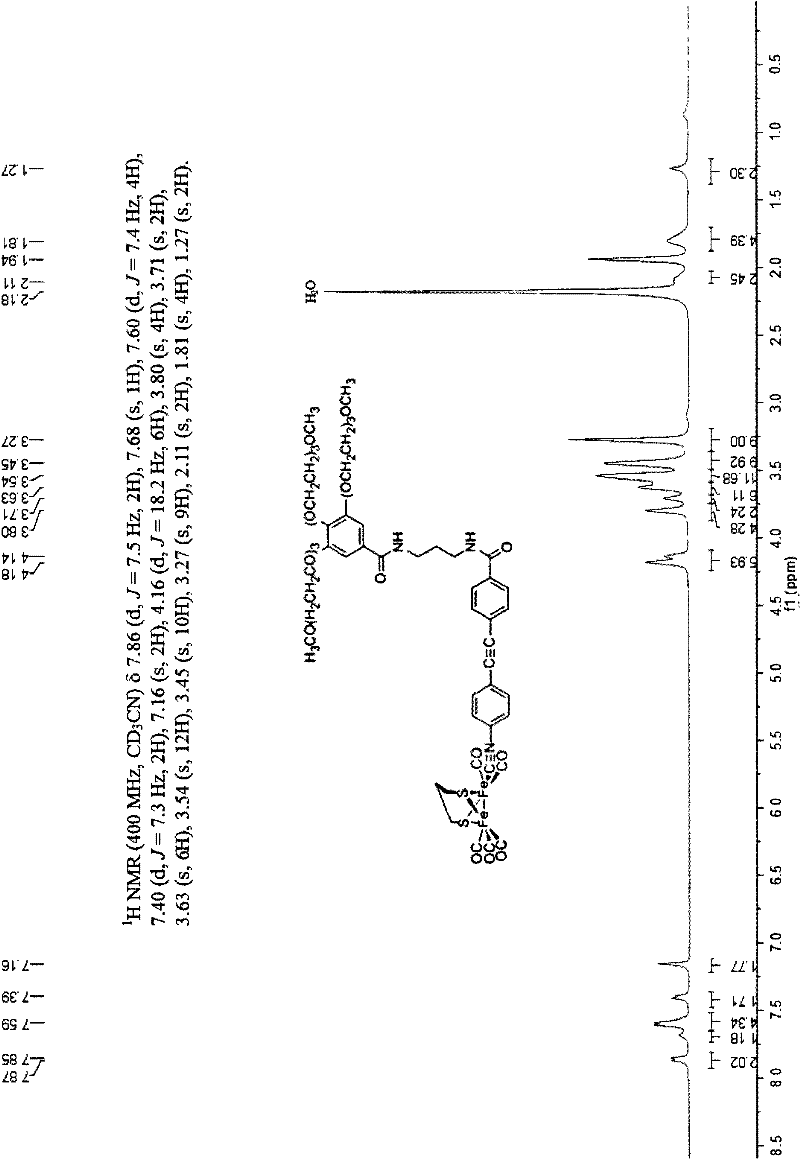
Photocatalytic hydrogen production system, method for preparing polycarbonyl diiron dithiolate cluster compound, and method for producing hydrogen
A polycarbonyl diiron disulfide cluster, photocatalytic technology, applied in the direction of iron organic compounds, organic compounds/hydrides/coordination complex catalysts, chemical instruments and methods, etc., can solve system deactivation, loss of catalytic activity, The problem of low catalytic hydrogen production efficiency, etc., achieves the effect of high stability and high hydrogen production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] A photocatalytic hydrogen production system containing polycarbonyl diiron disulfide cluster compounds: the concentration of water-soluble polycarbonyl diiron disulfide cluster compounds is 1.56×10 -4 M; Concentration of CdTe quantum dots (referred to as: MPA-CdTe QDs) stabilized with mercaptopropionic acid (in terms of Cd 2+ Meter) is 1.00×10 -3 M; ascorbic acid (H 2 A) The concentration is 8.52×10 -2 M; the size of MPA-CdTe QDs is 3.4nm; the solvent is water; the total sample volume is 10ml; the pH of the sample before light is 4.0.
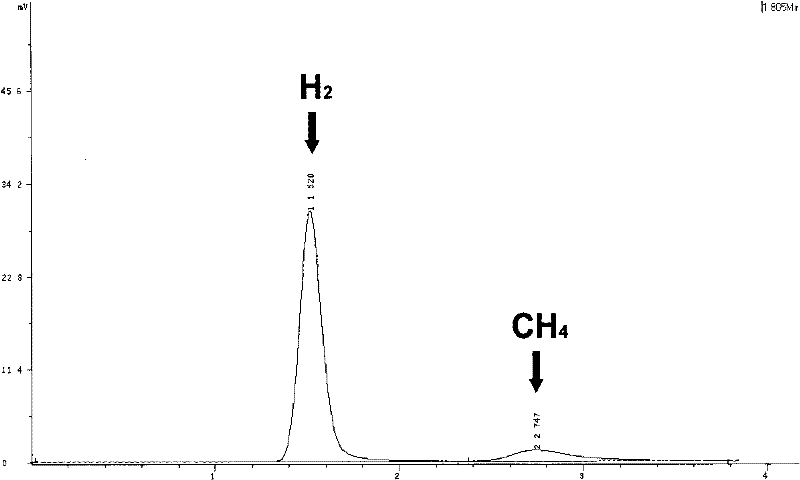
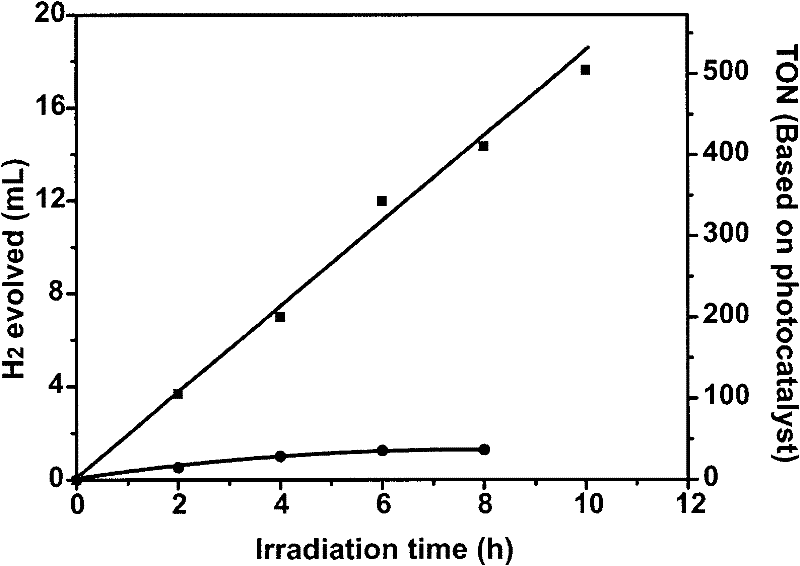
[0047] The method for preparing hydrogen by using the above-mentioned photocatalytic hydrogen production system is: use λ>400nm visible light to irradiate the sample, and monitor the sample once every 2 hours of illumination; after 10 hours of illumination, the calculated hydrogen production of the sample is about 17.6ml ( TON=505); the sample continued to produce hydrogen in the first ten hours, indicating that the system was stable ...
Embodiment 2
[0051] A photocatalytic hydrogen production system containing polycarbonyl diiron disulfide cluster compounds: the concentration of water-soluble polycarbonyl diiron disulfide cluster compounds is 1.56×10 -4 M; concentration of MPA-CdTe QDs (as Cd 2+ Meter) is 5.00×10 -4 M; ascorbic acid (H 2 A) The concentration is 8.52×10 -3 M; the size of MPA-CdTeQDs is 3.4nm; the solvent is water; the total sample volume is 10ml; the pH of the sample before light is 2.0.
[0052] The method of preparing hydrogen by using the above photocatalytic hydrogen production system is: irradiate the sample with visible light of λ>400nm, and monitor the sample by gas spectrometer every 2 hours of light; after 8 hours of light, the calculated hydrogen production of the sample is about 0.12ml (TON=3); the sample continued to produce hydrogen in the first six hours, indicating that the system was basically stable during the first six hours of light.
[0053] The polycarbonyl diiron disulfide cluster...
Embodiment 3
[0055] A photocatalytic hydrogen production system containing polycarbonyl diiron disulfide cluster compounds: the concentration of water-soluble polycarbonyl diiron disulfide cluster compounds is 1.56×10 -4 M; Concentration of MPA-CdTe QDs (as Cd 2+ Meter) is 5.00×10 -4 M; ascorbic acid (H 2 A) The concentration is 8.52×10 -3 M; the size of MPA-CdTeQDs is 3.4nm; the solvent is water; the total sample volume is 10ml; the pH of the sample before light is 3.0.
[0056] The method for preparing hydrogen by using the above-mentioned photocatalytic hydrogen production system is: irradiate the sample with visible light of λ>400nm, and monitor the sample by gas spectrometer every 2 hours of light; after 8 hours of light, the calculated hydrogen production of the sample is about 1.01ml (TON=29); the sample continued to produce hydrogen in the first six hours, indicating that the system was basically stable during the first six hours of light.
[0057] The polycarbonyl diiron disul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com