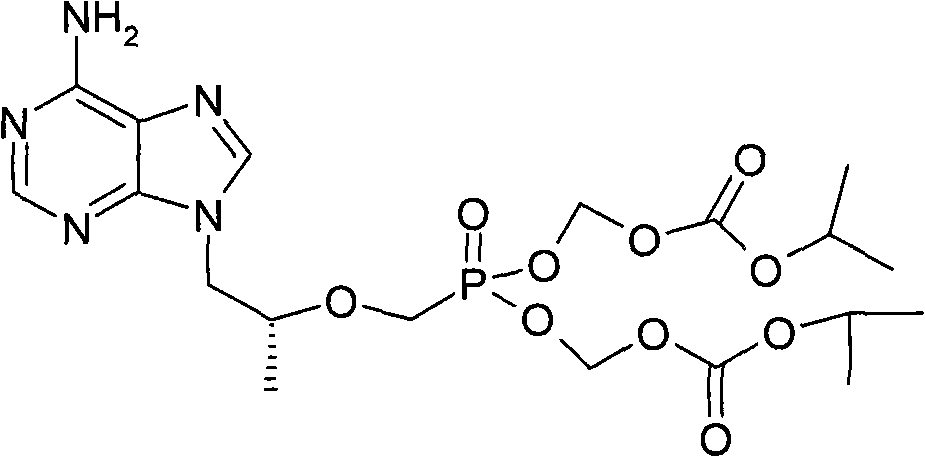
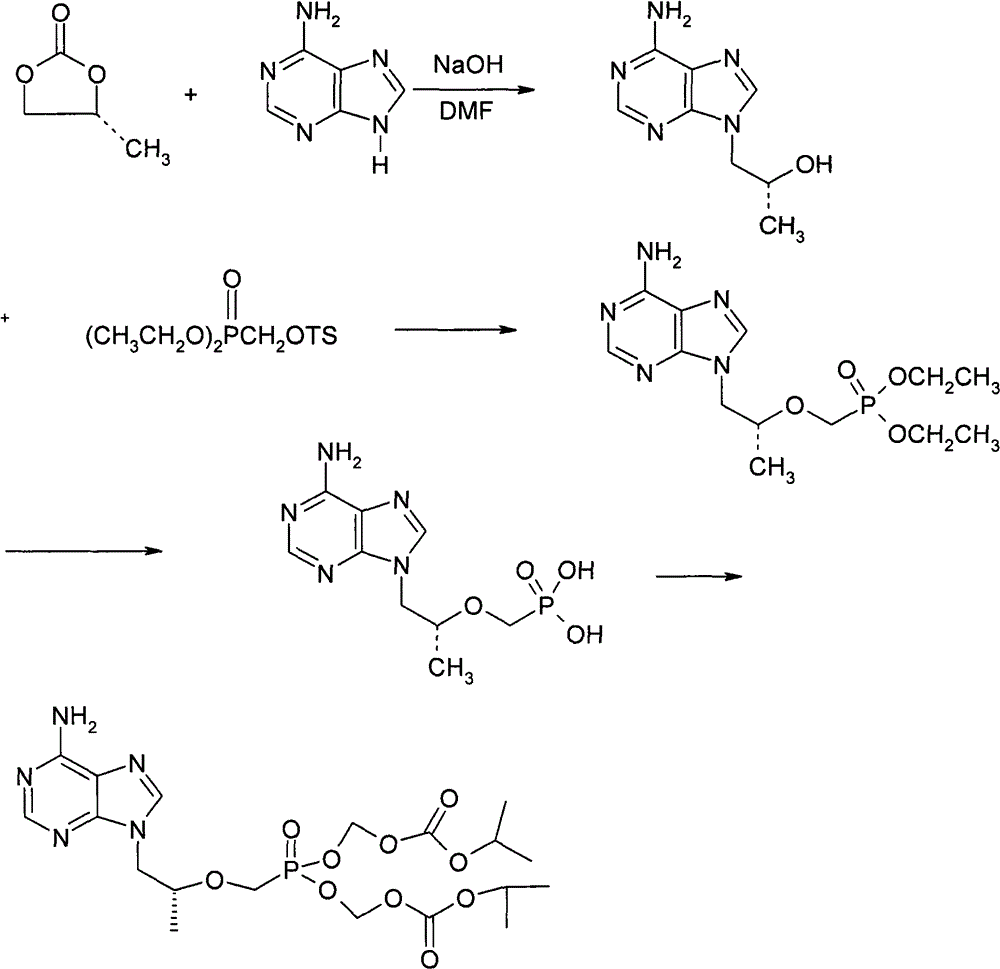
Method for preparing (R)-9-(2-phosphorylmethoxypropyl)adenyl-di(isopropoxycarbonylmethyl)ester
A technology of chloromethyl isopropyl carbonate and tenofovir dipivoxil, which is applied in the field of synthesis of 9-adenine diester, can solve the problems of lower reaction conversion rate and incomplete condensation reaction, and achieve easy source easy to control process operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Under argon protection, 50.0 g of PMPA (6.8% moisture content) was added to 150 ml of acetonitrile, stirred at room temperature for 5 minutes, 60 ml of triethylamine was added, and most of the solids were dissolved. The temperature was raised to 50°C and stirring was continued for 30 minutes. Then the temperature was raised to 75°C, and the solvent was distilled off under reduced pressure, and the measured moisture content of the residue was 0.8%. Cool down to 50°C, add 200 ml of N-methylpyrrolidone, 38 ml of triethylamine, and 55.0 g of tetrabutylammonium bromide to the residue, and stir for 30 minutes. Add 100ml of chloromethyl isopropyl carbonate dropwise within 30 minutes, and keep warm for 4 hours after dropping. After the reaction was completed, it was cooled to below 20°C with an ice bath, and the reaction mixture was added dropwise to 1500 ml of brine at 0°C. Stir until solidified, stir for another 5 hours, and filter. The filter cake was dissolved with 600ml...
Embodiment 2
[0036] Under argon protection, 10.0 g of PMPA (6.8% moisture content) was added to 20 ml of acetonitrile, stirred at room temperature for 5 minutes, 12 ml of triethylamine was added, and most of the solids were dissolved. The temperature was raised to 60°C and stirring was continued for 30 minutes. Then the temperature was raised to 75°C, and the solvent was distilled off under reduced pressure, and the measured moisture content of the residue was 1.6%. Cool down to 60°C, add 40ml of N-methylpyrrolidone and 7.6ml of triethylamine to the residue, and stir for 30 minutes. Add 20ml of chloromethyl isopropyl carbonate dropwise within 30 minutes, and keep it warm for 4 hours after dropping. After the reaction was completed, it was cooled to below 20°C with an ice bath, and the reaction mixture was added dropwise to 300 ml of brine at 0°C. Stir until solidified, stir for another 5 hours, and filter. The filter cake was dissolved with 150 ml of isopropyl acetate and washed with wa...
Embodiment 3
[0038] Under argon protection, 10.0 g of PMPA was added to 80 ml of acetonitrile, stirred at room temperature for 5 minutes, 12 ml of triethylamine was added, and most of the solids were dissolved. The temperature was raised to 50°C and stirring was continued for 30 minutes. Then the temperature was raised to 75° C., the solvent was evaporated under reduced pressure, and the measured moisture content of the residue was 0.5%. Cool down to 60°C, add 40ml of N-methylpyrrolidone and 12ml of triethylamine to the residue, and stir for 30 minutes. Add 20ml of chloromethyl isopropyl carbonate dropwise within 30 minutes, and keep it warm for 4 hours after dropping. After the reaction was completed, it was cooled to below 20°C with an ice bath, and the reaction mixture was added dropwise to 300 ml of brine at 0°C. Stir until solidified, stir for another 5 hours, and filter. The filter cake was dissolved with 150 ml of isopropyl acetate and washed with water. It was dried over anhydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com