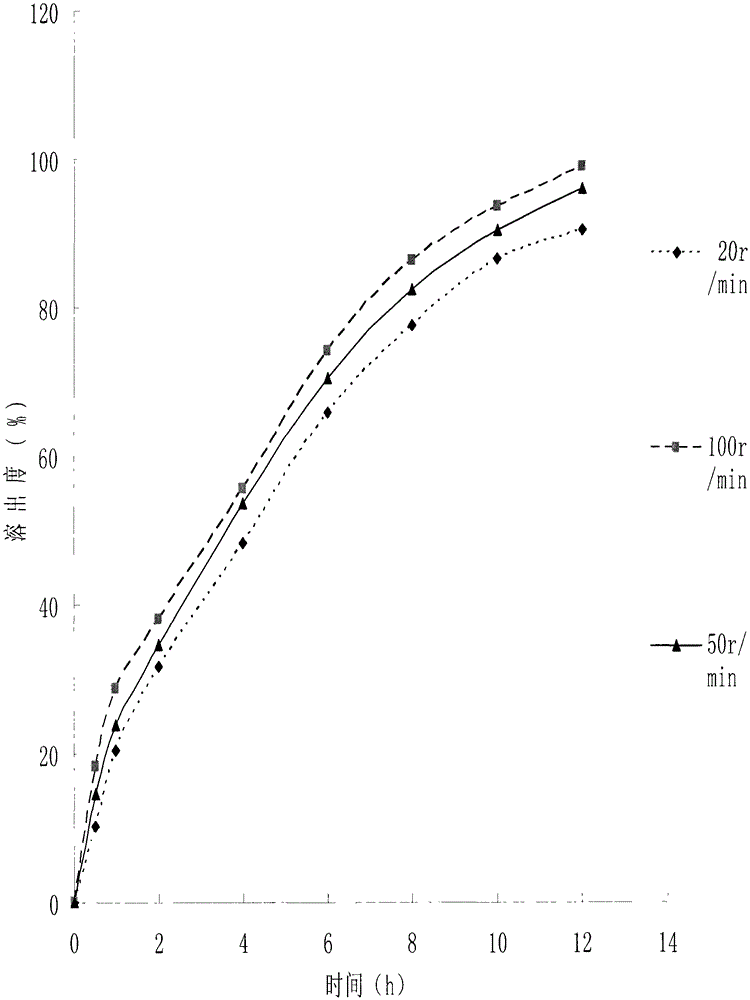
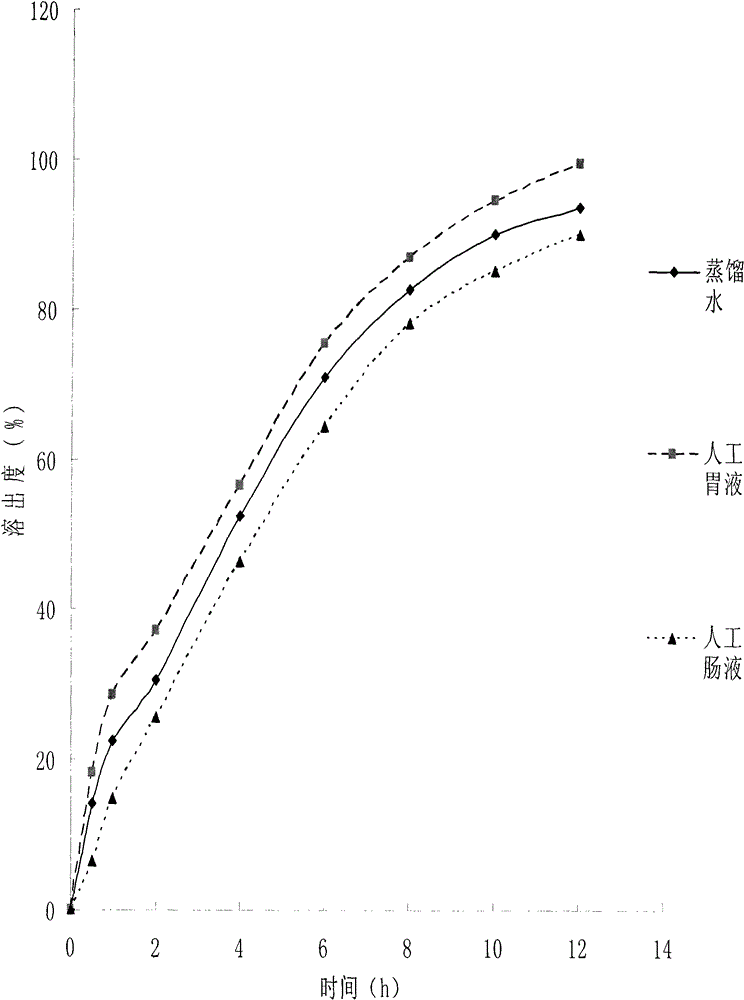
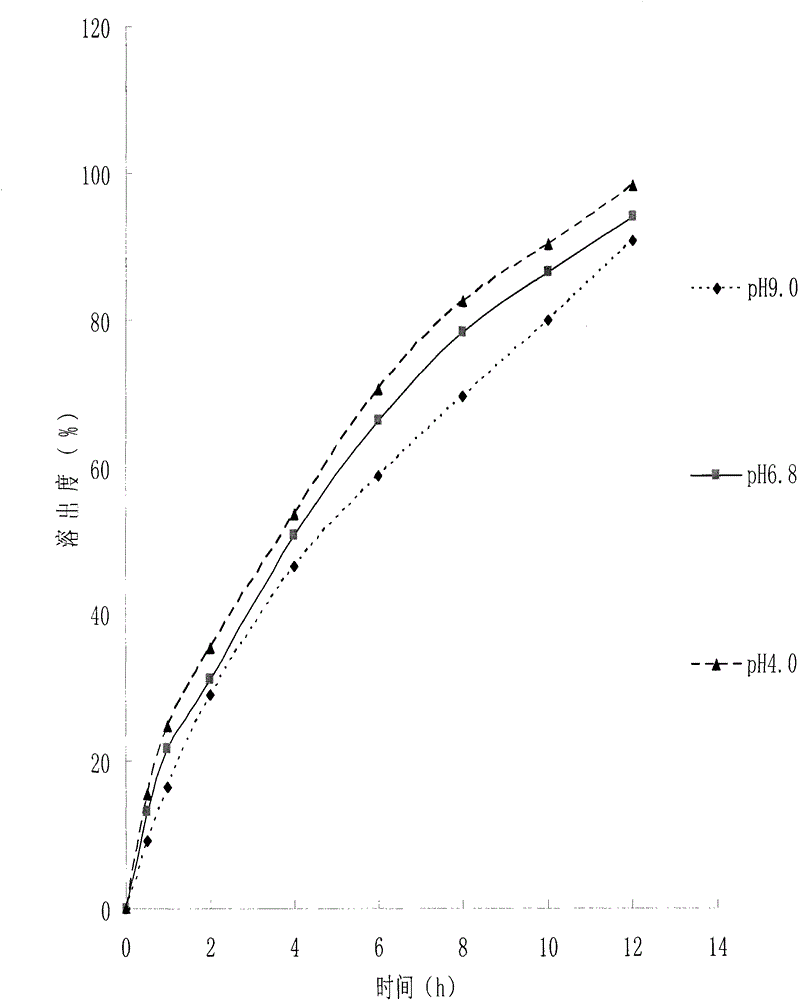
A kind of medicinal gelatin microsphere and preparation method thereof
A gelatin and microsphere technology, which is applied in the direction of making medicines into special physical or oral form devices, non-active components of polymer compounds, and bulk delivery, etc. inconvenience etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Trimetazidine Hydrochloride Gelatin Microspheres
[0024] 1 prescription
[0025]
[0026] 2 preparation steps
[0027] a. Emulsification: Weigh 0.596kg of trimetazidine hydrochloride and dissolve it in 440mL of 15% (w / v) gelatin solution, bathe in water at 60°C for 10 minutes, then slowly add the gelatin solution to preheat 60°C, and dissolve 3 % (w / v) in the liquid paraffin of mixed emulsifier, the oil-water volume ratio is 3: 1, and mixed emulsifier is made up of Span 80, 1% glyceryl monostearate by mass ratio (3: 1), while adding While stirring, the stirring speed is 200r / min, stirring for 20min;
[0028] b. Deoiling and dehydration: quickly cool the emulsion obtained in "a" to below 5°C in an ice-water bath, add 2000mL of a mixture of isopropanol and n-butanol, stir for 10min, and filter with suction;
[0029] c. Cross-linking and solidification: Take out the filter cake obtained in "b" and place it in a closed container, add 1000 mL of isopropanol solution c...
Embodiment 2
[0043] Trimetazidine Gelatin Microspheres
[0044] 1 prescription
[0045]
[0046] 2 preparation steps
[0047] a. Emulsification: Weigh 0.61kg of trimetazidine and dissolve it in 400mL of 20% (w / v) gelatin solution, bathe in water at 60°C for 15 minutes, then slowly add the gelatin solution to preheat 60°C, and dissolve 2% (w / v) In the liquid paraffin of the mixed emulsifier, the oil-water volume ratio is 4:1, and the mixed emulsifier is composed of Span 85 and Tween-20 according to the mass ratio (4:1), stirring while adding, the stirring speed 300r / min, stirring for 30min;
[0048] b. Deoiling and dehydration: quickly cool the emulsion obtained in "a" to below 5°C in an ice-water bath, add 1600mL of isopropanol, stir for 5min, and filter with suction;
[0049] c. Cross-linking and curing: take out the filter cake obtained in "b" and put it in a closed container, add 800mL of isopropanol solution containing 10% (v / v) formaldehyde, put it in an environment below 5°C for ...
Embodiment 3
[0063] Determination of Plasma Concentration of Trimetazidine Hydrochloride Sustained Release Gelatin Microspheres
[0064] Healthy subjects were divided into two groups, one group was given trimetazidine hydrochloride tablets sold on the market, one tablet every 8 hours, each containing 20 mg of medicine, and one group was given trimetazidine hydrochloride sustained-release gelatin microspheres, once every 12 hours, 0.1g each time, monitor the blood drug concentration for 24 hours, take 4ml of venous blood at 0.5, 1, 2, 2.5, 3, 6, 8, 12, 14, 16, 20, 24 points in total after taking the medicine, and measure Blood concentration, (see appendix Figure 7 )
[0065] Depend on Figure 7 , the plasma concentration of trimetazidine hydrochloride gelatin microspheres was more stable than that of trimetazidine hydrochloride tablets, and the difference between peak and trough was smaller, indicating that trimetazidine hydrochloride gelatin microspheres had sustained-release characteri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com