Method for preparing substituted spirooxazine photochromic compound
A photochromic and compound technology, applied in chemical instruments and methods, color-changing fluorescent materials, organic chemistry, etc., can solve the problems of low yield of photochromic compounds, expensive preparation cost, etc., and achieve bright color and low preparation cost. , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
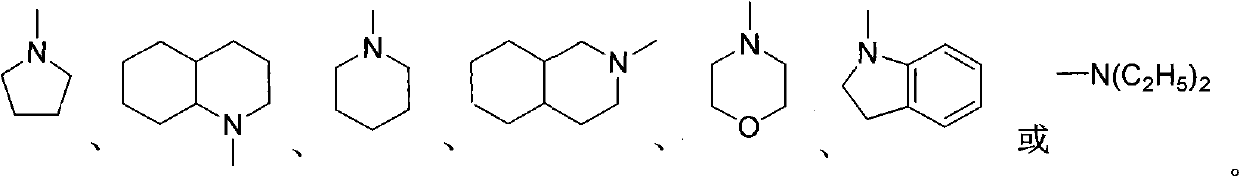
[0028] Preparation of photochromic compound 1,3,3-trimethyl-6'-tetrahydropyrrole-spiroindoline-2,3'-[3H]naphtho[2,1-b][1,4]oxa Zinc.
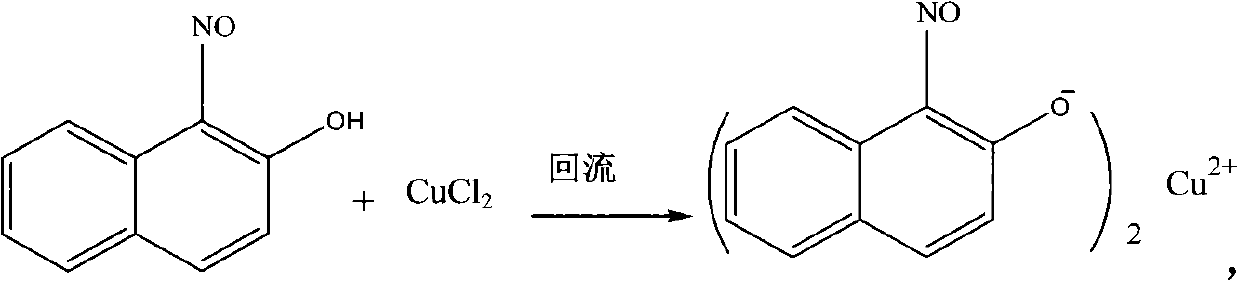
[0029] The first step, the preparation of 1-nitroso-2-naphthol copper complex
[0030] 1.36 g of CuCl 2 It is configured as the aqueous solution A that the mass percentage concentration is 30%, and 3.95 grams of 1-nitroso-2-naphthols are dissolved in the mixed solvent that the volume ratio is 1: 1 tetrahydrofuran and water, and configuration is that concentration is 0.04g / mL of solution B, as CuCl 2 The mass ratio of 1-nitroso-2-naphthol is 1:2.9, the solution A is added dropwise to the solution B under stirring, and the stirring is continued for 15 minutes, then suction filtered and dried to obtain the product 1- Nitroso-2-naphthol copper complex 3.91 g, yield 96%.
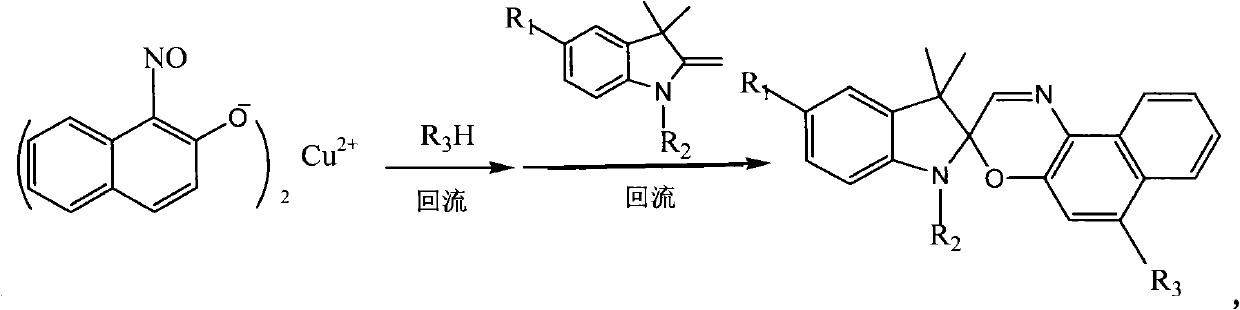
[0031] The second step, 1,3,3-trimethyl-6'-tetrahydropyrrole-spiroindoline-2,3'-[3H]naphtho[2,1-b][1,4]oxazine Synthesis
[0032] In a 100mL three-necked round-bottomed fla...
Embodiment 2
[0034] Preparation of photochromic compound 1,3,3-trimethyl-6'-morpholine-spiroindoline-2,3'-[3H]naphtho[2,1-b][1,4]oxazine .
[0035] The first step, the preparation of 1-nitroso-2-naphthol copper complex
[0036] With embodiment 1.
[0037] The second step, 1,3,3-trimethyl-6'-morpholine-spiroindoline-2,3'-[3H]naphtho[2,1-b][1,4]oxazine synthesis
[0038] In a 100mL three-necked round-bottomed flask, add 1mmol of the 1-nitroso-2-naphthol copper complex and 4mmol of morpholine prepared in the first step, and dissolve them in the 20mL of the three-necked round-bottomed flask first In ethanol, heat to reflux for 3 hours, then add 15 mL of 1,3,3-trimethyl-2-methyleneindoline ethanol solution with a concentration of 0.1 mmol / mL, continue to heat and reflux for 9 hours, monitor by TLC, stop the reaction Afterwards, use column chromatography to separate the photochromic compound 1,3,3-trimethyl-6'-morpholine-spiroindoline-2,3'[3H]naphtho[2,1-b The solid product of ][1,4]oxazine...
Embodiment 3
[0040] Preparation of photochromic compound 5-chloro-1,3,3-trimethyl-6'-tetrahydropyrrole-spiroindoline-2,3'-[3H]naphtho[2,1-b][1 , 4] Oxazine.
[0041] The first step, the preparation of 1-nitroso-2-naphthol copper complex
[0042] With embodiment 1.
[0043] In the second step, 5-chloro-1,3,3-trimethyl-6'-tetrahydropyrrole-spiroindoline-2,3'-[3H]naphtho[2,1-b][1, 4] Synthesis of oxazines
[0044] In a 100mL three-necked round-bottomed flask, add 1mmol of the 1-nitroso-2-naphthol copper complex prepared in the first step and 3.5mmol tetrahydropyrrole, and dissolve them in the three-necked round-bottomed flask first 20mL of ethanol, heated to reflux for 3 hours, then added 15mL of ethanol solution of 5-chloro-1,3,3-trimethyl-2-methyleneindoline with a concentration of 0.1mmol / mL, and continued to heat and reflux for 5 hours, TLC monitoring, after stopping the reaction, separated by recrystallization to obtain the photochromic compound 5 chloro-1,3,3-trimethyl-6'-tetrahydro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com