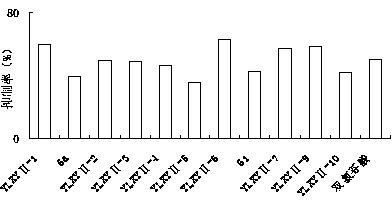Novel gas signal molecule donator and preparation method and use thereof
A compound and selected technology, applied in the field of medicine, can solve problems such as fatal cardiovascular events, achieve good safety and tolerance, and high pharmacological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
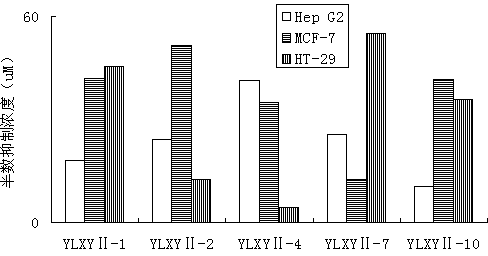
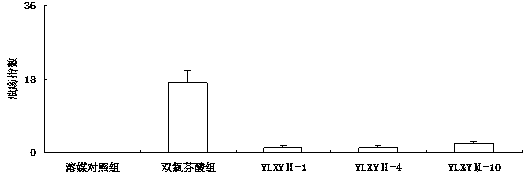
[0059] Example 2: E-2-(4-methoxyphenyl)-3-(4-methanesulfonylphenyl)-acrylic acid 4-(3H-1,2-dithio-hole-3- Synthesis of Thione-5-yl)-Phenyl Ester (YLXYⅡ-1)
[0060] Will 6a (0.203g, 0.61mmol), ADT-OH (0.152g, 0.67mmol) and BOP (0.323g, 0.73mmol) were added to 15mL of dry THF, then triethylamine (0.35mL, 2.44mmol) was added, stirred at room temperature 12h, then add 15mL ethyl acetate to dilute, then use 1 mol.L -1 Hydrochloric acid solution (3×15mL), saturated NaHCO 3 solution (3×15mL) and saturated brine (3×15mL), and the organic phase was washed with anhydrous MgSO 4 Drying, evaporation to dryness, column chromatography [petroleum ether (60-90):ethyl acetate=6:1 (v / v)], a red solid was obtained with a yield of 85.6%, mp: 72.1~73.1°C.
[0061] 1 H NMR (400MHz, CDCl 3 ), δ(ppm): 8.08 (s, 1H, =CH), 8.00(m, 3H, ArH), 7.83 (m, 3H, ArH), 7.42 (m, 3H, ArH & =CH), 7.28 (d , 2H, J = 8.7 Hz, ArH), 7.00 (d, 2H, J = 7.8 Hz, ArH), 3.77 (s, 3H, CH 3 ), 3.22 (s, 3H, CH 3 ).
...
Embodiment 3
[0065] Example 3: E-2-(2,4-dichlorophenyl)-3-(4-methylsulfonylphenyl)-acrylic acid 4-(3H-1,2-dithio-hole-3 Synthesis of -thione-5-yl)-phenyl ester (YLXYⅡ-2)
[0066] According to the synthetic method of YLXYⅡ-1, ADT-OH and 6b Preparation, red solid, yield 86.9%, mp: 179.1~180.1℃.
[0067] 1 H NMR (400MHz, CDCl 3 ), δ(ppm): 8.28 (s, 1H, =CH), 8.00 (d, 2H, J = 7.7 Hz, ArH), 7.90(m, 4H, ArH), 7.51 (d, 1H, J = 7.1 Hz, ArH), 7.40 (m, 4H, ArH), 6.87(s, 2H, ArH), 6.65(s, 1H, =CH), 3.22 (s, 3H, CH 3 ).
[0068] IR(KBr, cm -1 ): 1739.8 (C=O), 1490.9, 1521.8, 1637.6, 1595.1 (C=C), 1151.5 (C=S).
[0069] HR-MS: Calcd. For C 25 h 16 Cl 2 o 4 S 4 577.9309, Found: 577.9308.
Embodiment 4
[0070] Example 4: E-2-(2-chlorophenyl)-3-(4-methylsulfonylphenyl)-acrylic acid 4-(3H-1,2-dithio-hole-3-thione -5-yl)-phenyl ester (YLXYⅡ- 3 )Synthesis
[0071] According to the synthetic method of YLXYⅡ-1, ADT-OH and 6c Preparation, red solid, yield 81.5%, mp: 176.4-177.4°C.
[0072] 1 H NMR (400MHz, CDCl 3 ), δ(ppm): 8.25 (s, 1H, =CH), 8.01 (d, 2H, J = 8.7 Hz, ArH), 7.83 (m, 3H, ArH & =CH), 7.67 (d, 1H, J = 8.1 Hz, ArH), 7.50 (m, 1H, ArH), 7.42(m, 4H, ArH), 7.37 (d, 2H, J = 2.0 Hz, ArH), 3.22 (s, 3H, CH 3 ).
[0073] 13 C NMR (400MHz, CDCl 3 ), δ(ppm): 220.887, 177.937, 169.575, 158.848, 147.066, 146.892, 143.851, 141.339, 138.985, 138.507, 136.970, 136.844, 136.227, 136.141, 135.213, 134.522, 134.258, 133.484, 132.519, 128.340, 48.500.
[0074]IR(KBr, cm -1 ): 1734.0 (C=O), 1654.9, 1635.6, 1598.9, 1489.0 (C=C), 1151.5 (C=S).
[0075] HR-MS: Calcd. For C 25 h 17 ClO 4 S 4 545.9669, Found: 545.9661.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com