Amyloid fibrillar oligomer conformational epitope polypeptide and application thereof
A technology of amyloid protein and antibody, applied in the biological field, can solve problems such as patient death, achieve the effect of eliminating side effects and good immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1, the preparation of polypeptide PEOS and derivative thereof and antibody
[0050] 1. Obtaining Peptide PEOS
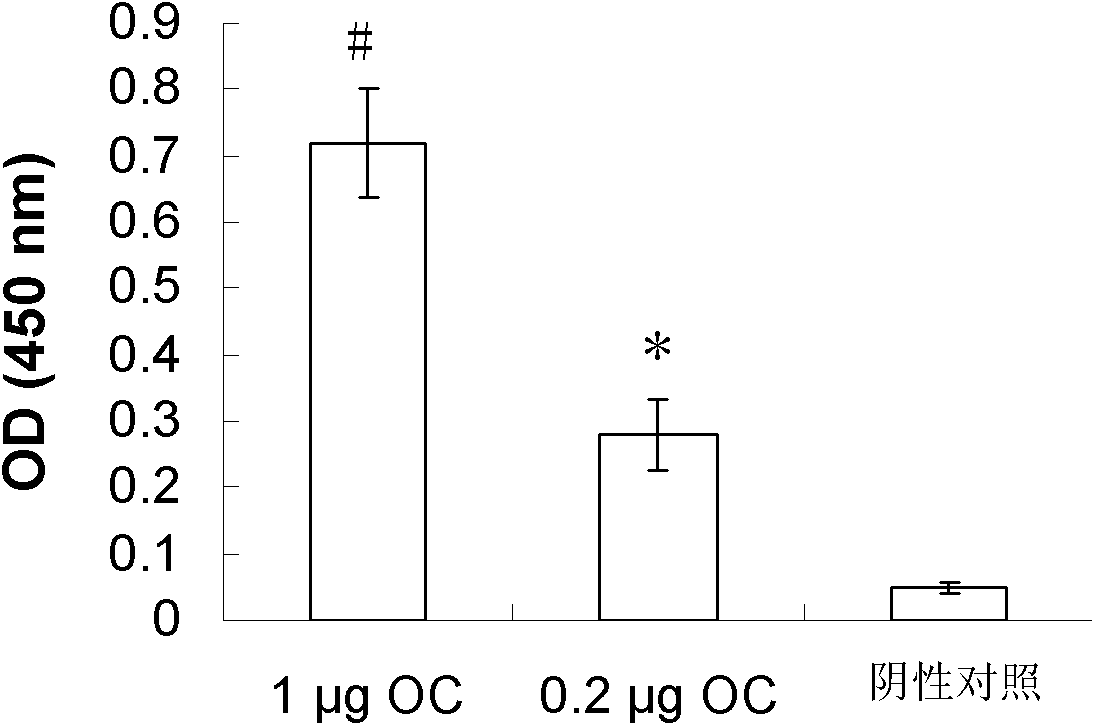
[0051] The present invention uses phage display technology, with the polyclonal antibody OC (Millipore, USA, catalog number AB2286) that specifically recognizes fibrous oligomers and fibers as a screening substrate, and 1 × 10 8 A circular heptapeptide was panned for four rounds, and phage clones that could significantly bind to OC were screened by phage ELISA, and obtained by amino acid sequencing. Polypeptide PEOS, its amino acid sequence is: Cys-Trp-Thr-Thr-His-Gln-Arg-Ser-Cys (sequence 1, CWTTHQRSC)
[0052] The synthetic preparation method of PEOS is:
[0053] PEOS is a cyclic peptide, plus 2 cysteines at both ends to form a 9-peptide, which can be artificially synthesized and prepared by Shanghai Gil Biochemical Co., Ltd. according to conventional methods (see Weng C C, Peter D W. Fmoc Solid Phase Peptide for the preparation method Synthesi...
Embodiment 2
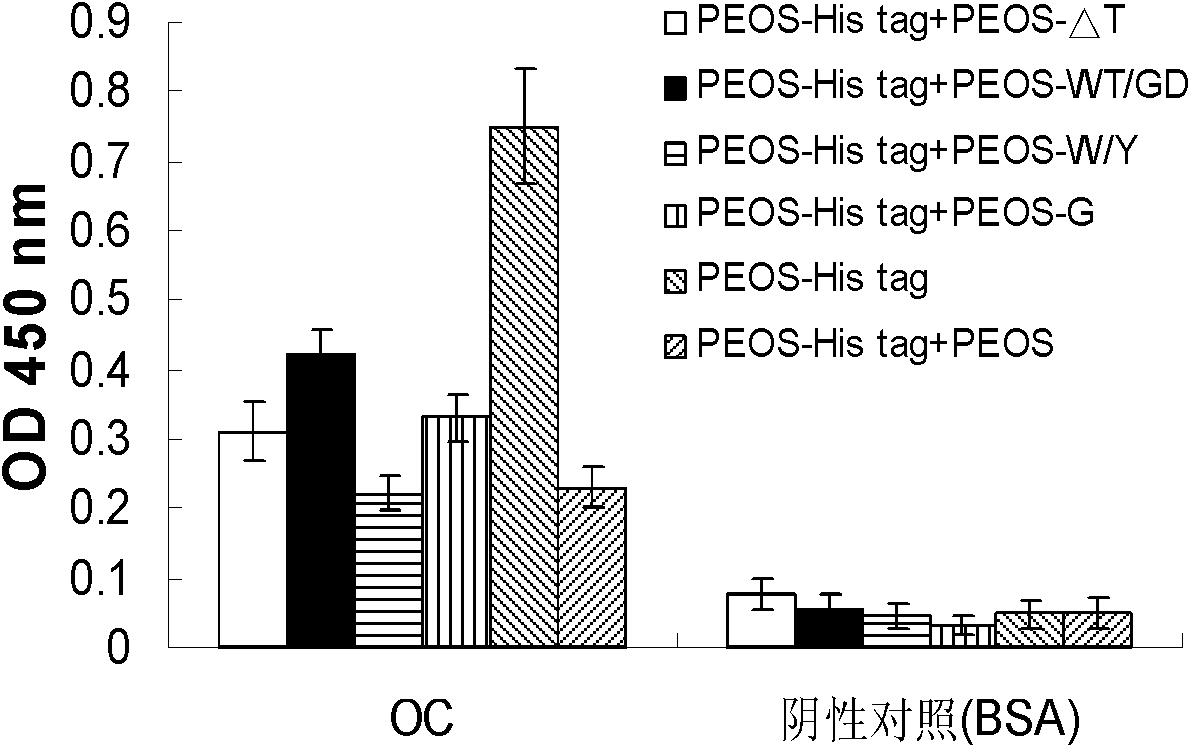
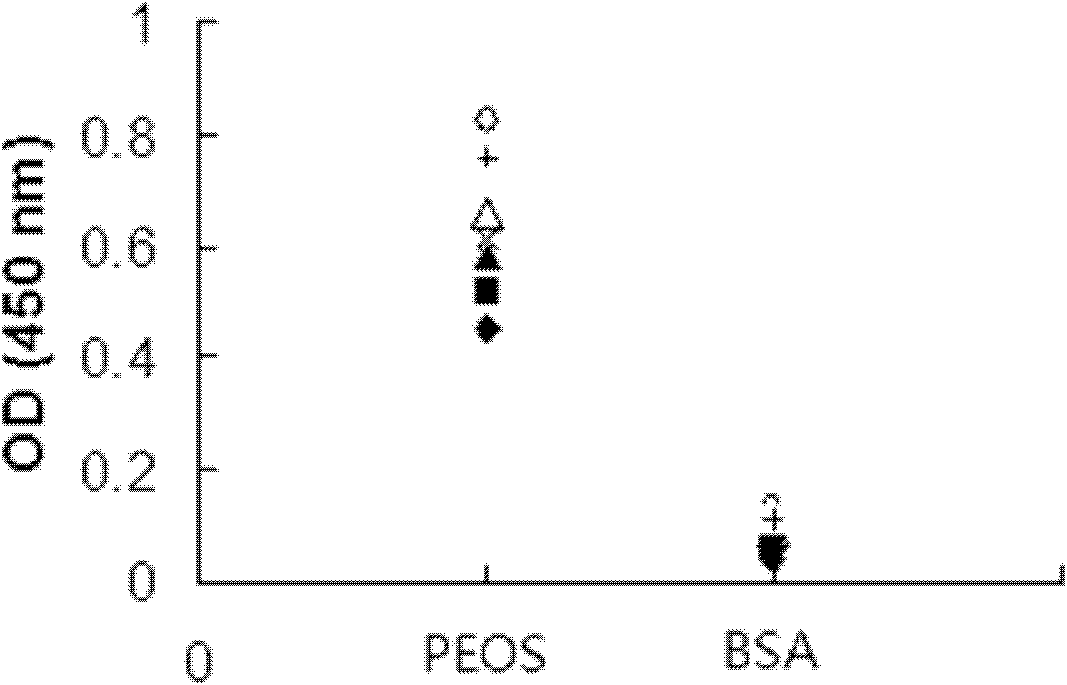
[0070] Embodiment 2, the application of polypeptide PEOS and its antibody
[0071] 1. Application of PEOS antibody
[0072] 1. Binding of PEOS antibody to various amyloid fibrillar oligomers and fibers
[0073] The purchased Aβ42 (American Peptide Co., catalog number 62-0-80B), amylin (American Peptide Co., catalog number 74-5-14B), α-synuclein (American rpeptide, catalog number No. S-1001-2), PrP (American Peptide Co., catalog No. 62-0-07B) were treated as follows: dissolved in hexafluoroisopropanol (HFIP) to 1 mg / ml, at room temperature (25°C) Sonicate for 10 minutes, dispense into epidorf tubes, volatilize HFIP in a vacuum environment, and store at -20°C. Before use, place the HFIP-treated Aβ42, amylin, and α-synuclein at room temperature for 20 minutes, add dimethyl sulfoxide (DMSO) to make the concentration of each protein 5 mg / ml, and then use 0.02M PBS buffer solution with pH 7.4 Dilute to desired concentration. PrP was prepared directly with 0.02M pH 7.4 PBS to the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com