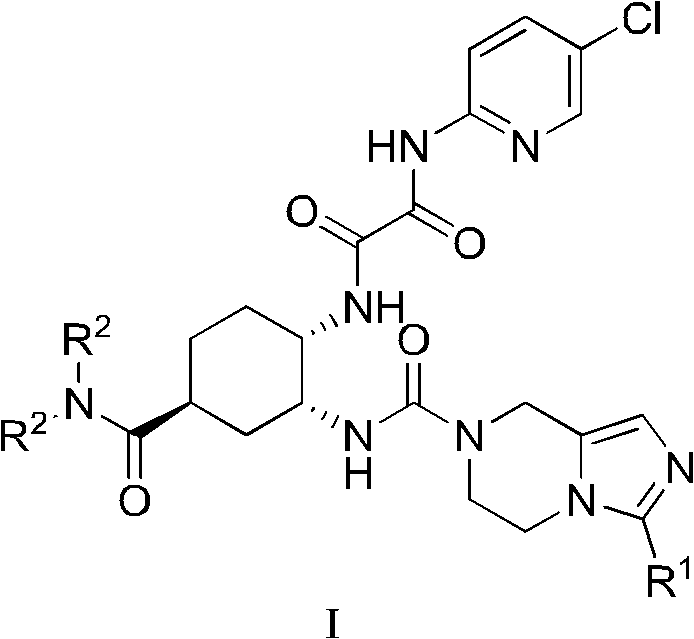
Anticoagulant diamine derivative
A technology of oxalamide, compounds, applied in the field of new compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 (1S, 3R, 4R)-3-[(tert-butoxycarbonyl)amino]-4-hydroxyl-N,N-dimethylcyclohexylformamide
[0037]
[0038] Dissolve 10 mL of 5 g (1S, 3S, 6R)-N, N-dimethyl-7-oxabicyclo[4.1.0] heptane-3-carboxamide in ethanol, add 30 mL of 28% ammonia water at room temperature, and raise the temperature Stirring at 40°C for 24 hours, the reaction was complete; the reaction mixture was concentrated under reduced pressure to obtain (1S,3R,4R)-3-amino-4-hydroxy-N,N-dimethylcyclohexylformamide crude product 5.9g, the crude product Dissolve in 25mL of water, add 9.65g of di-tert-butyl dicarbonate and 7g of sodium bicarbonate at room temperature, stir and react at 40°C for 2 hours, the reaction is complete, extract 3 times with 25mL of methyl isobutyl ketone, combine the organic phases, Concentrate under reduced pressure to obtain a crude product, which is recrystallized from methyl isobutyl ketone, filtered, and dried to obtain 6.3 g of the title compound.
[0039] 1 H-NMR (CDCl...
Embodiment 2
[0041] Example 2 (1S, 3R, 4R)-3-[(tert-butoxycarbonyl)amino]-4-methanesulfonyloxy-N,N-dimethylcyclohexylformamide
[0042]
[0043] At room temperature, 21.5g (1S,3R,4R)-3-[(tert-butoxycarbonyl)amino]-4-hydroxy-N,N-dimethylcyclohexylformamide was dissolved in 200mL methyl isobutyl ketone Add 17g of triethylamine and 16g of methanesulfonyl chloride in sequence, stir at room temperature for 1 hour, and the reaction is complete; add water to the reaction solution, separate the liquids, and concentrate the organic phase under reduced pressure to obtain a crude product. Add 75 mL of methyl isobutyl ketone to the crude product , stirred at room temperature for 3 hours, filtered, and dried to obtain 24.3 g of the title compound.
[0044] 1 H-NMR (CDCl 3 )δ: 1.45(s, 9H), 1.59-1.66(m, 1H), 1.68-1.77(m, 1H), 1.85-1.96(m, 2H), 2.04-2.14(m, 1H), 2.16-2.27( m, 1H), 2.73-2.79(m, 1H), 2.93(s, 3H), 3.02(s, 3H), 3.08(s, 3H), 3.98-4.06(m, 1H), 4.65-4.83(m, 2H).
[0045] [α]=-15° (c=1.0,...
Embodiment 3
[0046] Example 3 (1S, 3R, 4S)-4-amino-3-[(tert-butoxycarbonyl)amino]-N,N-dimethylcyclohexylformamide oxalate
[0047]
[0048] At room temperature, 20 g of (1S, 3R, 4R)-3-[(tert-butoxycarbonyl)amino]-4-methanesulfonyloxy-N, N-dimethylcyclohexylformamide was dissolved in 100 mL of toluene, Add 7.14g of sodium azide and 6.5g of benzyltriethylammonium chloride in turn, raise the temperature to 60°C and stir for 72 hours, then the reaction is complete; add water to the reaction solution, separate the liquids, wash the organic phase with saturated brine and water successively, and directly Add methanol for dilution, then add 7.5% Pd / C and ammonium formate, stir at 40°C for 1 hour, TLC monitors that the reaction is complete; after filtering to remove Pd / C, the organic phase is concentrated under reduced pressure to obtain a crude product, which is dissolved in 200 mL of aqueous acetonitrile and 4.94 g of oxalic anhydride, stirred at room temperature for 17 hours, filtered to obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com