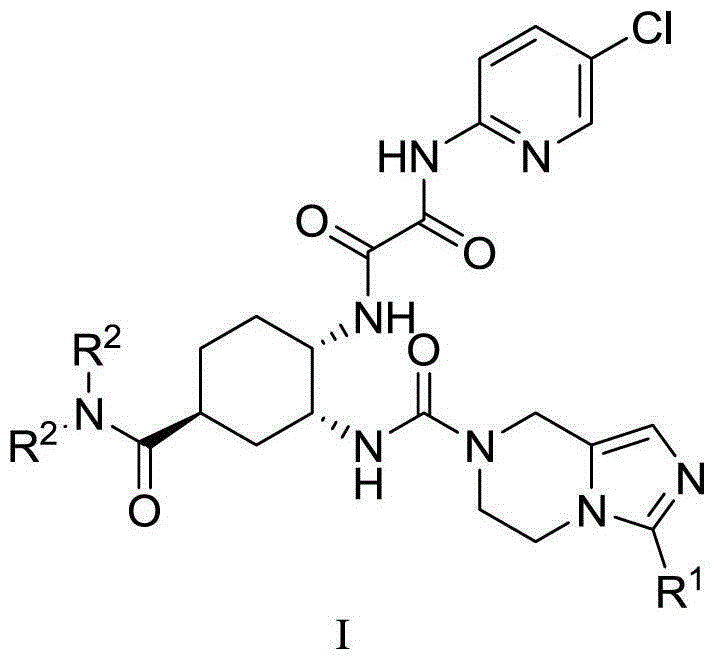
Anticoagulation diamine derivative
A compound, tert-butoxycarbonyl technology, applied in the field of new compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 (1S,3R,4R)-3-[(tert-butoxycarbonyl)amino]-4-hydroxyl-N,N-dimethylcyclohexylformamide
[0037]
[0038] Dissolve 10mL of 5g (1S,3S,6R)-N,N-dimethyl-7-oxabicyclo[4.1.0]heptane-3-carboxamide in ethanol, add 30mL of 28% ammonia water at room temperature, and heat up to Stirring at 40°C for 24 hours, the reaction was complete; the reaction mixture was concentrated under reduced pressure to obtain 5.9 g of the crude product of (1S,3R,4R)-3-amino-4-hydroxy-N,N-dimethylcyclohexylformamide, and the crude product was Dissolve in 25 mL of water, add 9.65 g of di-tert-butyl dicarbonate and 7 g of sodium bicarbonate at room temperature, stir and react at 40°C for 2 hours, the reaction is complete, extract 3 times with 25 mL of methyl isobutyl ketone, combine the organic phases, Concentrate under reduced pressure to obtain a crude product, which is recrystallized from methyl isobutyl ketone, filtered, and dried to obtain 6.3 g of the title compound.
[0039] 1 H-NMR (C...
Embodiment 2
[0041] Example 2 (1S,3R,4R)-3-[(tert-butoxycarbonyl)amino]-4-methanesulfonyloxy-N,N-dimethylcyclohexylformamide
[0042]
[0043] At room temperature, 21.5g (1S,3R,4R)-3-[(tert-butoxycarbonyl)amino]-4-hydroxy-N,N-dimethylcyclohexylformamide was dissolved in 200mL methylisobutyl To the ketone, add 17g triethylamine and 16g methanesulfonyl chloride successively, stir at room temperature for 1 hour, and the reaction is complete; after adding water to the reaction solution, separate the liquids, and concentrate the organic phase under reduced pressure to obtain a crude product, to which 75mL methyl isobutyl Ketone, stirred at room temperature for 3 hours, filtered, and dried to obtain 24.3 g of the title compound.
[0044] 1 H-NMR (CDCl 3 )δ:1.45(s,9H),1.59-1.66(m,1H),1.68-1.77(m,1H),1.85-1.96(m,2H),2.04-2.14(m,1H),2.16-2.27( m,1H),2.73-2.79(m,1H),2.93(s,3H),3.02(s,3H),3.08(s,3H),3.98-4.06(m,1H),4.65-4.83(m, 2H).
[0045] [α]=-15°(c=1.0, CHCl 3 ).
Embodiment 3
[0046]Example 3 (1S,3R,4S)-4-amino-3-[(tert-butoxycarbonyl)amino]-N,N-dimethylcyclohexylformamide oxalate
[0047]
[0048] Dissolve 20g of (1S,3R,4R)-3-[(tert-butoxycarbonyl)amino]-4-methanesulfonyloxy-N,N-dimethylcyclohexylformamide in 100mL of toluene at room temperature , add 7.14g of sodium azide and 6.5g of benzyltriethylammonium chloride successively, raise the temperature to 60°C and stir for 72 hours, then the reaction is complete; add water to the reaction solution, separate the liquids, and wash the organic phase with saturated brine and water successively, Directly add methanol for dilution, then add 7.5% Pd / C and ammonium formate, stir at 40°C for 1 hour, TLC monitors that the reaction is complete; after filtering to remove Pd / C, the organic phase is concentrated under reduced pressure to obtain a crude product, which is dissolved in 200 mL of aqueous acetonitrile and 4.94 g of oxalic anhydride, stirred at room temperature for 17 hours, filtered to obtain a cry...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com