Anthropogenic LMP1 (latent membrane protein1) extracellular regional antibody for nasopharyngeal carcinoma and application thereof
A technology for the extracellular region and nasopharyngeal carcinoma, which is applied in the field of genetic engineering and immune-targeted diagnosis and treatment, and can solve the problem of lack of human anti-nasopharyngeal carcinoma LMP1 extracellular region antibody
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
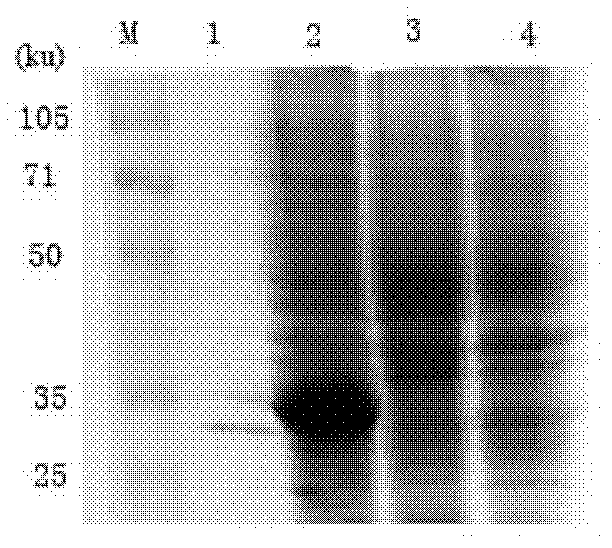
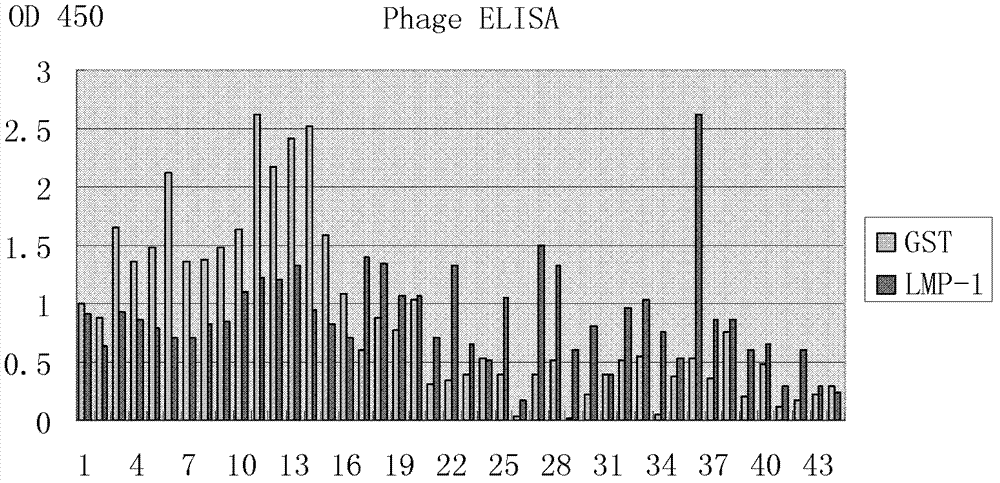
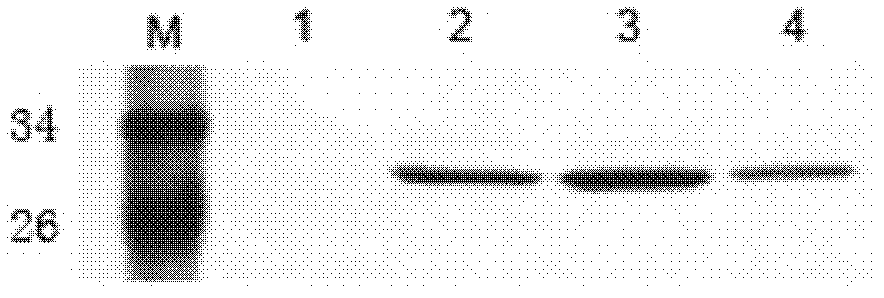
[0030] Example 1 Preparation and Identification of Human Anti-LMP1 Extracellular Region Antibody Fab
[0031] 1. Preparation and purification of latent membrane protein 1 (LMP1) extracellular region antigen
[0032] 1. The gene sequence of the extracellular region of LMP-1 comes from the data published by GenBank. The three extracellular regions of LMP-1 LMP-1[46-50]DWTGG, [98-103]WNLHGQ, and [161-166]QQNWW are spliced into WNLHGQ WNLHGQ QQNWW QQNWW DWTGG WNLHGQ WNLHGQ QQNWW QQNWW DWTGG (SEQ ID NO.5); gene sequence is 5'-GC GGATCC TGG AAC CTG CAC GGT CAG TGG AAC CTG CAT GGT CAA CAG CAA AAC TGG TGG CAA CAA AAC TGG TGG GAC TGG ACC GGT GGC TGG AAC CTG CAC GGT CAG TGG AAC CTG CAC GGC CAG CAG CAA AAC TGG TGG CAG CAG AAC TGG TGG GAT TGG ACT GGC GGT TGA GAATTC G-3' (SEQ ID NO.6); and the restriction sites (underlined) of BamH I and EcoR I were designed at the 5' end and 3' end respectively, and the whole gene was synthesized by Nanjing Saibaisheng Biological Co., Ltd., and Th...
Embodiment 2
[0210] Example 2: Preparation of HLEAFab-MMC immunotoxin and its in vivo and in vitro effects on nasopharyngeal carcinoma cells
[0211] 1. Preparation of HLEAFab-MMC immunotoxin
[0212] 1. MMC was reacted with n-hydroxysuccinimide 3-(2-pyridinedimercapto)propionate (SPDP, Pierce, Rockford, IL) at 4°C for 2 hours, and then placed at -80°C for use.
[0213] 2. HLEAFab was reduced with 2-iminosulfane hydrochloride in pure nitrogen, and the reduced antibody was purified using a desalting column (Pierce, Rockford, IL).
[0214] 3. The activated HLEAFab and the activated mitomycin (Mytomycin C, MMC) were mixed and reacted (HLEAFab:Mytomycin C molar ratio=1:1). React for 1 hour at room temperature in pure nitrogen, and remove excess MMC-SPDP using a desalting column.
[0215] 4. HLEAFab-MMC products are sterilized by filtration with a 0.2 μm ultrafiltration membrane (Pall, Ann Arbor, MI).
[0216] 5. Cell ELISA (the specific steps are the same as the HLEAFab cell ELISA detection...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com