Application of pimavanserin in preparation of antitumor drugs
An anti-tumor drug, the technology of pimaserin, applied in the field of medicine, can solve the problems of insufficient understanding, neonatal malformation, and the safety has not been well verified, and achieves the effect of good safety and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
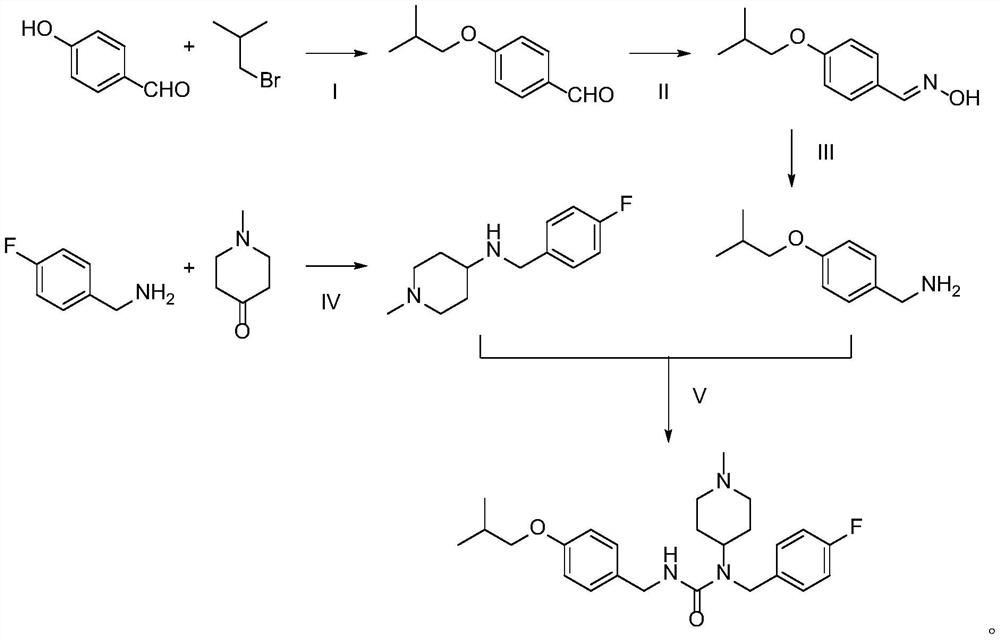
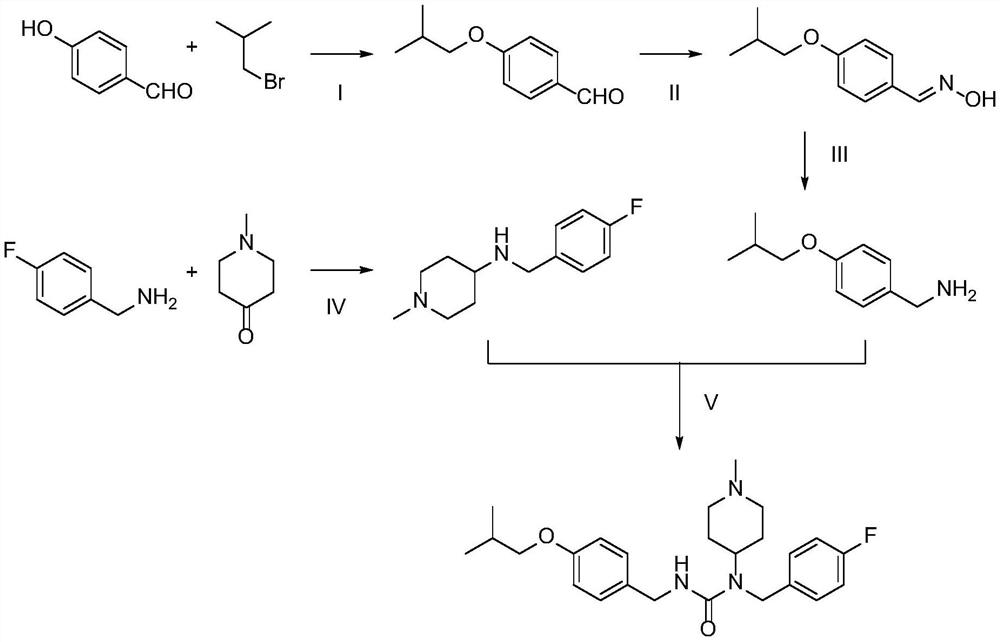
[0021]Example 1: Preparation of pirazine forest
[0022] Step i: Take 4-hydroxybenzaldehyde to remove hydroxybenzaldehyde (12.2 g, 100 mmol), bromine isobutane (13.7 g, 100 mmol), K 2 CO 3 (69 g, 500 mmol) was placed in a 250 mL flask, and 100 ml of DMF was added, and the reaction was stirred at 80 degrees for 24 h. The reaction was stopped, poured into 800 ml of water, stir until K 2 CO 3 Dissolve, EA was extracted 3 times, combined with an organic phase and washed with 2 mol / l NaOH solution, water, saturated brine, dried over anhydrous sodium sulfate, and evaporated, to give a pale yellow liquid to isobutyl isryaldehyde 12.23 g, The yield was 68.7%.
[0023] Step II: Sodium hydrogencarbonate (4.62 g, 55 mmol), hydroxylamine (3.82 g, 55 mmol) was placed in a flask, and 30 ml of water was added, and the reaction was stirred at room temperature for 20 min to give a hydroxylamine aqueous solution. It is proud to be placed in a flask (8.91 g, 50 mmol) to the flask, and 50 ml of EtO...
Embodiment 2
[0027] Example 2: Pirazinelin inhibits the inhibitory activity of A549, MCF7, HCT116, PC3 cells
[0028] (1) Experimental materials
[0029] Cell lines: A549, MCF7, HCT116, PC3 cells were lahed in 96-well plates at 1500, 2200, 800, and 2000 / well, 100 ul, 24 h, respectively, 24 h.
[0030] 马 色 林: Diluted with DMSO, diluted with a culture medium to be prepared to be stored at -20 ° C for five different concentrations of 5 μm, 2.5 μm, and the final concentration of DMSO is below 0.1%.
[0031] Positive control: SorafeniB, 5-fluorouracil (5-Fluorouracil).
[0032] MTT: The PBS was dissolved to 2 mg / ml, stored at -20 ° C.
[0033] (2) Experimental method
[0034] With the MTT method, the A549, MCF7, HCT116, PC3 cells were selected to evaluate the anti-tumor value of the test sample. A549, HCT116, and PC3 cell line were cultured on RPMI 1640 medium, which contains 10% calf serum (FBS), and MCF-7 cell lines were cultured on DMEM medium, which contains 10% calf. Serum (FBS). When the ...
Embodiment 3
[0039] Example 3: Tablet formula
[0040] Active compound 25-1000 mg, starch 45 mg, microcrystalline cellulose 35 mg, polyvinylpyrrolidone (10% aqueous solution) 4 ml, sodium carboxymethylcellulose 4.5 mg, stearate 0.5 mg, talc 1 mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com