Method for preparing organic carboxylate from olefin through hydroesterification
A technology of organic carboxylate and olefin hydrogen ester, which is applied in the preparation of carbon monoxide or formate, chemical instruments and methods, organic chemistry, etc., can solve the problems of increased investment cost, complicated post-processing, equipment corrosion, etc., and achieve structural Can be designed to promote high-efficiency, low-corrosion effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
[0029] In a 60ml autoclave, sequentially add Pd(OAc) 2 : 0.01mmol, PPh 3 : 0.04mmol, acidic ionic liquid a: 0.1mmol, anhydrous methanol: 10mL, seal the reactor, replace the reactor 3 times with carbon monoxide, charge ethylene: 2g (66.7mmol), and then charge carbon monoxide until the reactor pressure is 4.0MPa. The temperature was controlled by the temperature controller to slowly rise to 80°C, react for 4 hours, cool to room temperature, and unload the kettle. The liquid obtained from the reaction was qualitatively analyzed by Agilent 6890 / 5973 GC and quantitatively analyzed by Agilent 6820 gas chromatography. Ethylene The conversion rate was 98%, and the selectivity of the product methyl propionate was 99%.
Embodiment 2
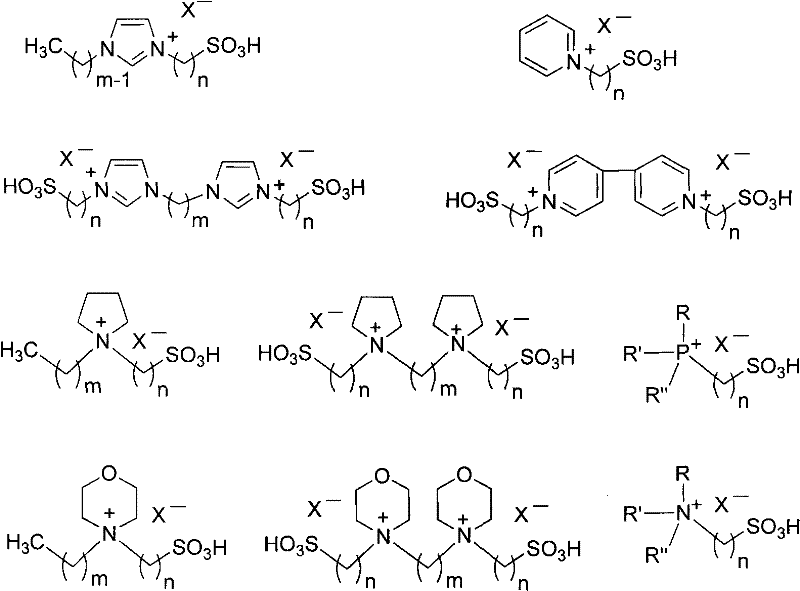
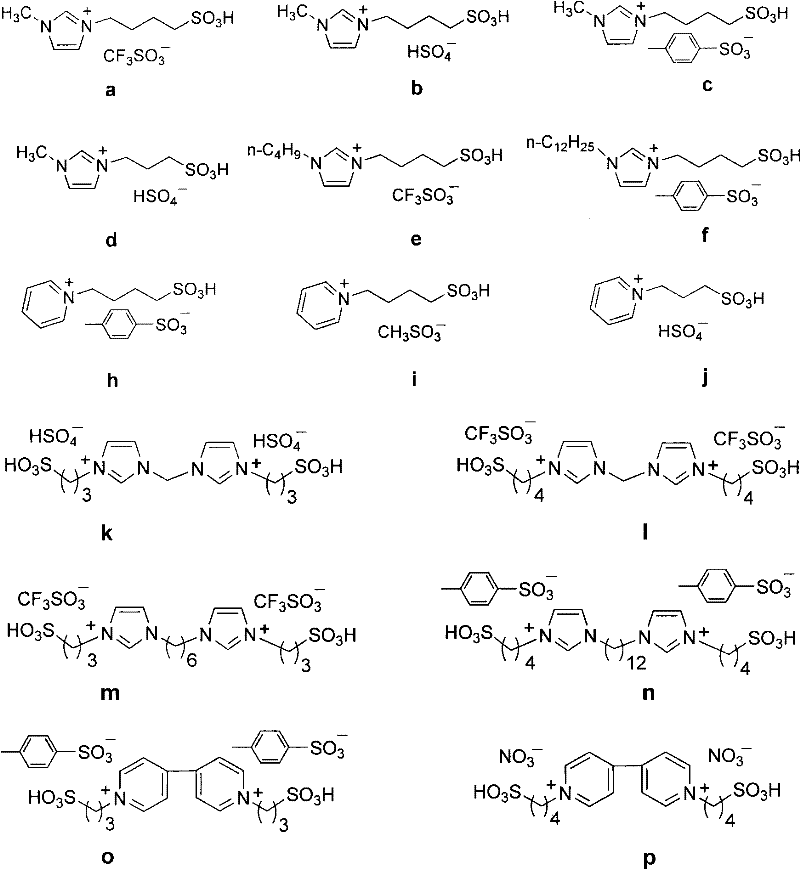
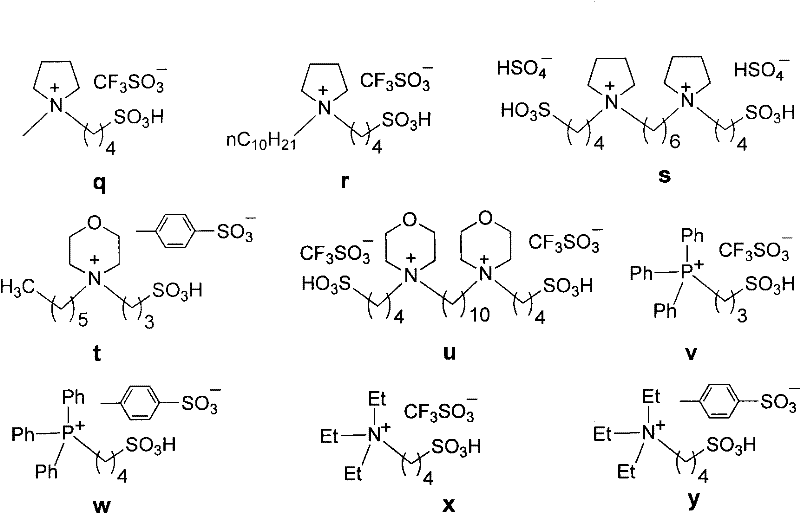
[0031] With embodiment 1, acidic ionic liquid promotor used is b-y (wherein the single nucleic acid ionic liquid consumption is 0.1mmol, and the binuclear ionic liquid consumption is 0.05mmol), reaction result is shown in the following table:
[0032]
[0033]
Embodiment 3
[0035] In a 60ml autoclave, sequentially add Pd(OAc) 2 : 0.01mmol, dtbpmb: 0.02mmol, acidic ionic liquid a: 0.1mmol, anhydrous methanol: 10mL, seal the reactor, replace the reactor with carbon monoxide for 3 times, fill with ethylene: 2g (66.7mmol), and then fill with carbon monoxide When the pressure of the reactor is 4.0 MPa, the reaction is carried out at 80° C. for 4 hours, the conversion rate of ethylene is 99%, and the selectivity of the product methyl propionate is 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com