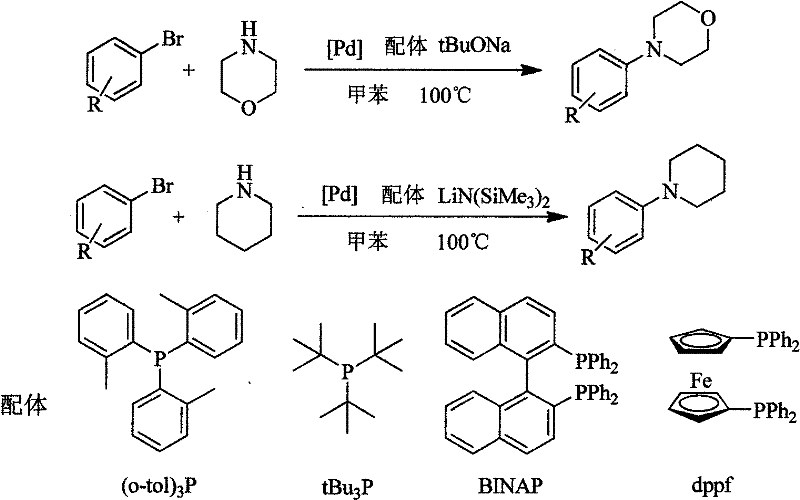
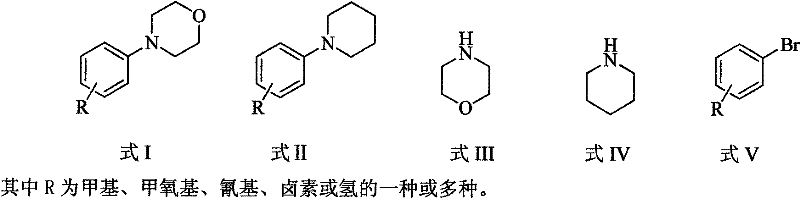
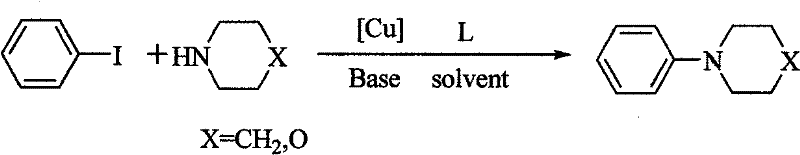Method for easily preparing aryl morpholine and aryl piperidine
A kind of technology of aryl morpholine and aryl piperidine, which is applied in the field of preparing aryl morpholine and aryl piperidine to achieve the effect of high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0020] The preparation of implementation case 1N-phenylmorpholine
[0021] Under nitrogen protection, 144mg (1.5mmol) sodium tert-butoxide, 3.6mg (0.02mmol) palladium dichloride, 10.6mg (0.04mmol) triphenylphosphine, 157mg (1.0mmol) bromobenzene were successively added to a 25ml Schlenk bottle , 130.5mg (1.5mmol) morpholine, 5.0ml toluene, heating to reflux for 5h, adding an appropriate amount of water to quench the reaction, extracting with ethyl acetate (3×10ml), combining the organic phases, concentrating, and separating by column chromatography to obtain White solid 141.7 mg, yield 88.0%. mp 49-51°C. 1 HNMR (400MHz, CDCl 3 ) δ 3.07 (t, J = 4.7Hz, 4H), 3.77 (t, J = 4.7Hz, 4H), 6.89-6.73 (m, 3H), 7.18-7.20 (m, 2H).
Embodiment 2
[0022] The preparation of embodiment 2 N-4-methylphenylmorpholine
[0023] The reaction substrate was replaced with 171 mg (1.0 mmol) of 4-methylbromobenzene, and the rest of the experimental conditions and operations were the same as in Example 1 to obtain 153.1 mg of a light yellow solid with a yield of 86.0%; mp 48-50°C. 1 H NMR (400MHz, CDCl 3 )δ2.20(s, 3H), 3.03(t, J=4.8Hz, 4H), 3.78(t, J=4.8Hz, 4H), 6.76(d, J=8.5Hz, 2H), 7.01(d, J=8.5Hz, 2H).
Embodiment example 3
[0024] Implementation Case 3 Preparation of N-4-chlorophenylmorpholine
[0025] The reaction substrate was replaced with 191.5 mg (1.0 mmol) of 4-chlorobromobenzene, and the remaining experimental conditions and operations were the same as in Example 1 to obtain 175.6 mg of a white solid with a yield of 88.9%. mp 65-68°C. 1 H NMR (400MHz, CDCl 3 )δ3.04(t, J=4.8Hz, 4H), 3.78(t, J=4.8Hz, 4H), 6.75(d, J=8.9Hz, 2H), 7.14(d, J=8.9Hz, 2H) .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com